Editor’s note: This text-based course is a transcript of the webinar, Functional Electrical Stimulation for OTs: Principles and Application, presented by Rebecca Martin, OTR/L, OTD, CPAM, CKTP.
Learning Outcomes
- After this course, participants will be able to recognize the therapeutic applications for using electrical stimulation.
- After this course, participants will be able to identify contraindications and precautions for electrical stimulation.
- After this course, participants will be able to describe the physiological mechanisms for muscle contraction when using electrical stimulation.
- After this course, participants will be able to select alterable parameters to adjust for specific patient care.
Introduction
Thank you so much for having me. I hope everybody is safe and healthy, and I am so glad that you made the decision to join me here today. Today, we are going to talk about functional electrical stimulation, specifically some considerations for occupational therapists. It is not a modality that we use a lot and can be a little bit scary I think for some people. Hopefully, after this course, you will walk away feeling like it is another tool in your tool bag. It is not always perfect, and you will not always know exactly the right answer. However, I do hope you learn how to problem-solve through it so that you can use it to help your clients.
NMES Assisted Grasp Training and Restoration of Function in the Tetraplegic Hand: A Case Study Series
Objective
- To determine the influence of repetitive NMES assisted grasp and release activities on the paretic tetraplegic hand.
First, I wanted to start with a little bit of evidence for you. This is a case study series that I published several years ago as part of my doctoral work. We looked at the influence of repetitive NMES assisted grasp and release activities on the paretic tetraplegic hand.
Participants
- Participant #1
- 21 y.o., female
- C5 ASIA A
- 21 mos. post-injury

Figure 1. Participant 1.
- Participant #2
- 17 y.o., male
- C5 ASIA C
- 6 mos. post-injury

- Participant #3
- 18 y.o., male
- C4 ASIA A
- 12 mos. post-injury

Figure 3. Participant 3.
We had three participants, the first was a 21-year-old female with C5 ASIA A 21 months post-injury; the second was a 17-year-old male who had a C5 ASIA C meaning that his injury was incomplete who was six months post-injury; and an 18-year-old male with C4 ASIA A, a complete injury who was 12 months post-injury. Our patients were all chronic, greater than six months out from their injuries, and all had cervical injuries. They all had sufficient shoulder motion to be able to reach anterior and across the midline, but they all had no hand function. Thus, they were not able to open or close their hands without assistance.
Intervention
This was the intervention shown in this video.
Video #1.

We applied sequential stimulation to the flexors and extensors. You can see when I let go of the trigger his hand closes, and when I push the trigger his hand opens. This assists the patient in grasping and then releasing these balls into the container. The balls varied in size and weight to prevent accommodation. We also moved the box from one side to the other so the patient would sometimes have to reach ipsilateral and sometimes contralateral, but it was always with a repetitive opening and closing of their hand.
We did each session for one hour. We did this specific intervention for 30 minutes and then did 30 minutes of functional skills training. Examples are stacking canes on a shelf, picking up and using a drinking cup, or any number of things. To give you a sense of how much practice this intervention involved, during the 30 minutes of that repetitive grasp and release training, the patients would on average open and close their hand 210 times. This was a huge volume of repetition as compared to other types of interventions.
Outcomes
We measured the patients with the Jebsen Taylor Hand Function Test, the Box and Blocks Test, dynamometer, and did a semi-structured interview at baseline immediately after the first session and after the final session.
Testing overview.
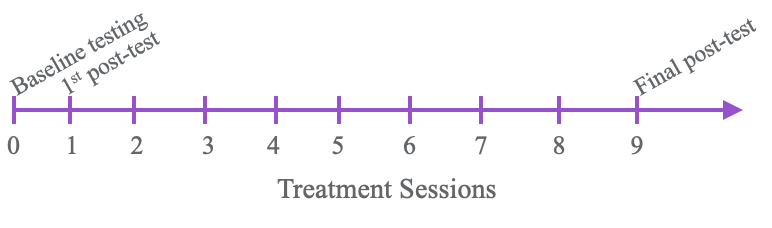
Figure 4. Overview of treatment sessions and testing.
Box and Blocks Test.
We did this over a two-week period so there were 10 sessions in total. Thankfully, we made things better for our clients. Figure 5 shows the results of the Box and Blocks Test.
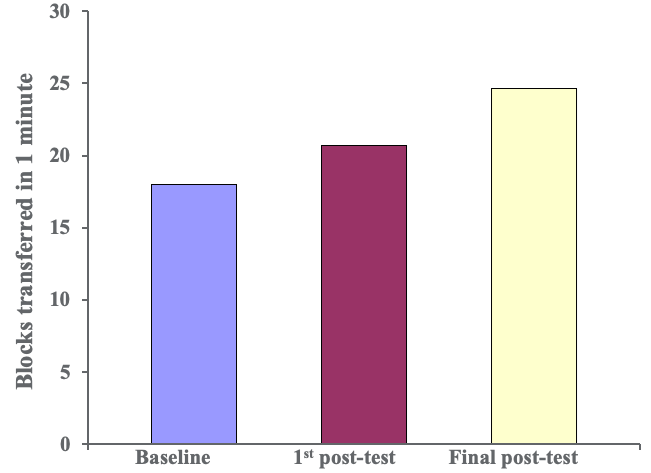
Figure 5. Results of the Box and Blocks Test.
This is an indirect measurement of how quickly clients can open and close their hands. As you can see, patients improved their function over time. Next are specific grasp and release patterns from a subtest of the Jebsen Taylor Hand Function Test.
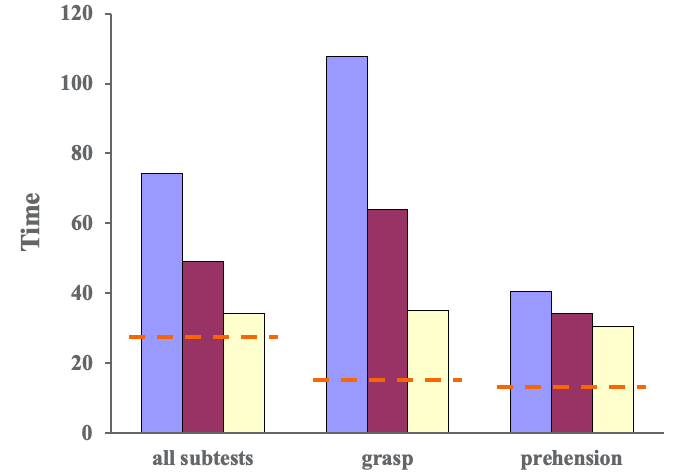
Figure 6. Results of the Jebsen Taylor Hand Function Test- grip and prehension patterns.
Some tests required a gross grasp and some require other patterns of prehension like tip or tripod pinch depending on the task. This was a timed test, and we wanted to see the time go down meaning that they got faster. If you look at all of the subtests, patients did pretty well. When we look at group averages, they decreased over time. This red dotted line indicates the age-based norms. If we separate that out and look at patterns that require a gross grasp, you can see that we made lots of significant changes. And, if you look at other patterns of prehension, tip with tripod pinch, for example, you can see that the change is more modest. This tells us there is some specificity of training related to what we did and not entirely a surprise. When we look specifically at the lifting large and heavy items on the Jebsen Taylor Test, we can see that patients made good gains in grip strength in Figure 6.
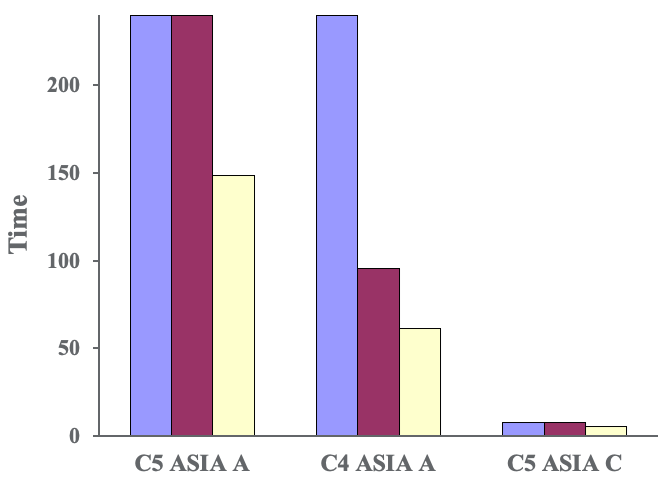
Figure 7. Jebsen Taylor Hand Function Test-grasp.
Our first two patients were not able to do it at all at baseline but did improve their performance after even just one session and more improvement after each session. Our final subject, with the incomplete injury, got a little bit worse before he got a bit better. When we went back and looked at what he was actually doing, he was using his spasticity to jam his hand around the can to lift it. By the end of the study, he was actually using a true grasp and release to pick up the can.
Subjective Patient Report
- “The treatment reminded me I have fingers.”
- “My fingers feel much looser like I can use them now.”
- “This weekend I picked up a full soda can and did not spill it!”
There is plenty of evidence to show that NMES is associated with increased cortical representation, and we saw that in the patients' use of and awareness of their hands and fingers. They were also able to engage in activities in social settings and felt much more confident in their hand skills.
Conclusions
- Improvements were observed in all main outcome measures.
- Most significant improvements were seen in grasp functions.
- Participants reported a reduction of spasticity, a more effective hand grasp, and greater endurance in functional tasks of the trained hand.
We saw improvements in all main outcome measures, and we saw the most significant improvements in grasp. Participants reported the reduction of spasticity, a more effective hand grasp, and greater endurance when completing functional tasks. In just two weeks, we made statistically significant meaningful improvements in patients' hand function with the use of a high-volume NMES training.
Indications and Precautions
Hopefully, now I have convinced you that electrical stimulation is useful. Now, we will talk about how you do it. As therapists, we like to use acronyms and sometimes we get lazy and use them interchangeably. It is important to define them as they are distinct and separate.
Types of Electrical Stimulation
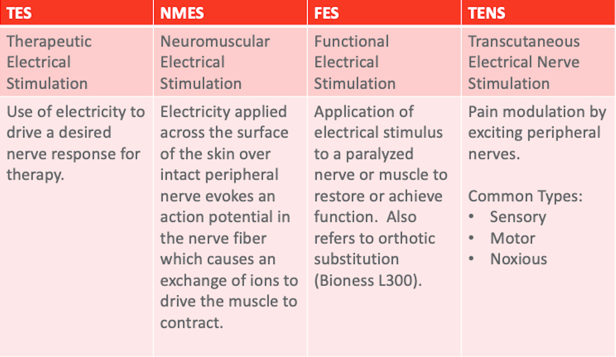
Figure 8. Types of electrical stimulation.
The first is therapeutic electrical stimulation or TES. This is the use of electricity to drive the desired nerve response for any kind of therapy. Basically, everything we do falls under this category of TES. As we start to drill down and get more specific, we first look at NMES. This is electricity applied across the surface of the skin to an intact peripheral nerve to drive a particular motor response. As the name neuromuscular implies, we are stimulating the nerve to get a muscle response. I think that is an important distinction that a lot of therapists miss. And, I do it as well. I talk about stimming a muscle. What I want is muscle action, but I am actually stimulating the nerve. Functional electrical stimulation is that same stimulation, but now it is used in a functional pattern. For example, if I were to stim a patient's wrist extensors in a nonspecific, repetitive way, it would be NMES. If I paired it with a functional task related to grasp and release or self-feeding, now it becomes functional and FES. To be honest, we should always be doing FES. AOTA came out with guidelines about interventions that should be avoided and modalities were on the list. However, as you read that a little bit closer as part of the Choosing Wisely campaign, you see that they are modalities without functional skill. What we are talking about today is incorporating this modality into functional treatment. Finally, TENS, or transcutaneous electrical nerve stimulation, is a pain modulation stimulation. We will talk about that in a few minutes, but it is a totally different stimulation paradigm than NMES and FES. A lot of patients say, "My mom has this stim unit from her back surgery 20 years ago. Can I use the same stimulator?" While you can turn a TENS unit up high enough to get a muscle response, this is less than ideal and we will talk about why in a moment.
Therapeutic Indications
- Increase circulation
- Reduce muscle spasm
- Promote healing of fracture or tissue
- Reduce edema
- Strengthening
- Improve and maintain muscle mass during or following periods of inactivity
- Maintain/gain ROM
- Re-educate/facilitate voluntary contraction
- Reduce the effects of spasticity
- Prevent/reverse disuse atrophy
- Orthotic substitution
We use electrical stimulation to increase circulation, reduce muscle spasms, promote healing of fracture or tissue, reduce edema, strengthen, and improve and maintain muscle mass during or following periods of inactivity. It can also be used to maintain or gain range of motion, re-educate and facilitate voluntary contraction, reduce the effects of spasticity, prevent and reverse disuse atrophy, and as an orthotic substitution. I like to think about my stim as sort of an extra set of hands or a way to supercharge what the patient is currently doing. If the patient has some initiation of the bicep, and you are doing a grooming task, you might use stim to help supercharge their biceps so they can reach their mouth or the top of their head. It is also helpful to think about it as an extra set of hands for a client sitting on the edge of the bed in preparation for ADLs. They may need just a little bit more trunk stability. Instead of using my hands, I can use stim to help stabilize their pelvis so that they can be stable, and then, I can use my hands elsewhere.
Contradictions/Precautions (at the discretion of the treating team)
- Implanted electrical device
- Active metastases
- Evidence of osteomyelitis
- Decreased sensation
- Thrombosis/hemorrhage
- Pregnancy
- Epilepsy
- Cognitive status
Of course, there are people for whom stimulation is not appropriate, but these contraindications and precautions should be at the discretion of the treating team. It really depends on the setting and the patient and how comfortable the team is. We should always have a conversation with our attending physician before treating someone. We tend to be pretty aggressive about how we use stimulation in my setting, but I understand that is not appropriate in everybody's setting. If somebody has an implanted electrical device, you want to make sure that the electricity you are delivering is not going to get in the way of the electricity associated with the device like cardiac pacemakers and defibrillators. You are fine to stimulate with a backup and pump around. In fact, we even stimulate in conjunction with diaphragmatic pacers because those are preset electrically driven devices versus a defibrillator, which is a sensing device. You do not want to stimulate patients with active metastases and definitely not over the site of the metastases. However, you would not tell a cancer patient to not exercise. The idea here is that an increase in circulation will cause an increase in metastases. But as I said, you would not tell a cancer patient to not exercise, and that is a much more significant increase in circulation. So if, for example, somebody has kidney cancer, you might not stim their paraspinals, but I would have no problems stimming their shoulder or their hand. You do not want to stim over evidence of osteomyelitis with the same idea that you do not want to increase circulation and spread of the infection. You also want to be careful over areas of decreased sensation making sure that a patient can feel or at least has a way to tell you if they are getting uncomfortable. That said, I stimulate patients with spinal cord injury who have no sensation all the time. I check their skin to make sure that I am not causing any irritation. You also do not want to increase thrombosis or hemorrhage, again, with an increase in circulation. We also do not want to stim those that are pregnant or have epilepsy. Lastly, with decreased cognitive status, we want to make sure that the client has a way to say that they are uncomfortable. If somebody is wearing a pain patch or some other medication patch, you would not stim over the area of the patch because the patch is not conducted. You could instead remove the patch, wipe off any medication that may remain on the skin, and then you would be fine to stimulate.
Neuroanatomy: A Quick Review
Time to dust off those neuroanatomy skills. I know you have them.
Figure 9 shows your brain and spinal cord.
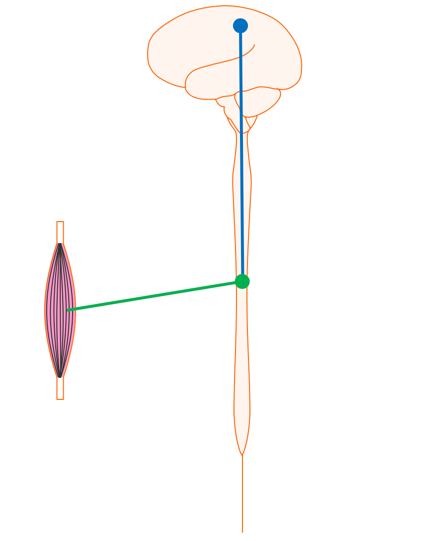
Figure 9. Brain and spinal cord.
The nerve that originates in the motor cortex of the brain and runs down the spinal cord to the level where it is needed is called the upper motor neuron. The nerve that runs from the spinal cord out to the muscle is called a lower motor neuron.
Upper Motor Neuron
- Nerve entirely in the central nervous system
- Cell body in the brain
- Axon in the spinal cord
- Damage results in
- Loss of movement and sensation
- Hyperreflexia
- Increased muscle tone
- Disuse atrophy
- Contracture secondary to increased tone
- Recovery is attributed to plasticity/redundancy but is probably a combination of factors (remyelination, endogenous stem cells, sprouting to other intact tracts)
The upper motor neuron is a nerve entirely contained in the central nervous system. It starts in the brain, and its axon runs through the spinal cord. When you have damage to this upper motor neuron, as is common in things like stroke, brain injury, and some parts of the spinal cord, you get an upper motor neuron presentation. This includes the loss of movement and sensation, hyperreflexia, increased muscle tone, disuse atrophy, and contracture secondary to increased tone. The upper motor neuron does not necessarily regrow, but recovery from upper motor neuron conditions like stroke and brain injury is attributed to plasticity and redundancy within the nervous system. There is probably also a combination of factors happening which includes some endogenous repair.
Lower Motor Neuron (LMN)
- The nerve which originates in the central nervous system ends in the peripheral nervous system
- Cell body in the spinal cord
- Axon outside the spinal cord (runs to a muscle)
- Damage results in
- Loss of movement and sensation
- Hyporeflexia
- Low to no muscle tone
- Denervation atrophy
- Contracture secondary to soft tissue shortening
- Damage to the axon only (in the extremity) regrows at the rate of 1cm/month
- Damage to the cell body (in the cord) does not regrow, may impact response to ES
The lower motor neuron originates in the central nervous system in the anterior part of the spinal cord and ends in the peripheral nerve at the neuromuscular junction. If you have damage to a lower motor neuron, as is the case in things like brachial plexus injury and spinal cord injury, you have damage to the anterior horn and have a loss of movement and sensation, hyporeflexia, low muscle tone, denervation atrophy, and contracture secondary to soft tissue shortening. With damage to a peripheral nerve, it will regrow. The Schwann cells are capable of delivering growth factors and directing axonal regrowth toward the neuromuscular end-plate. However, damage to the cell body, which is in the spinal cord, does not regrow. The distinction here is that that will impact your ability to deliver electrical stimulation.
Basic Electrophysiology
Mechanism for Contraction
- Electricity applied across the surface of the skin over an intact peripheral nerve evokes an action potential in the nerve fiber (like physiologically generated potentials) causing the muscle to contract.
To get a contraction with electrical stimulation, electricity is delivered across the surface of the skin over an intact peripheral nerve to evoke an action potential and causes a muscle to contract (Figure 10).
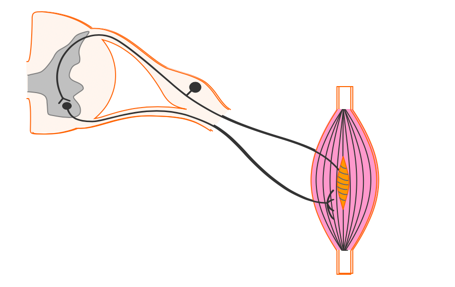
Figure 10. Stimulating over a peripheral nerve to elicit a response in the muscle spindle.
Video #2.
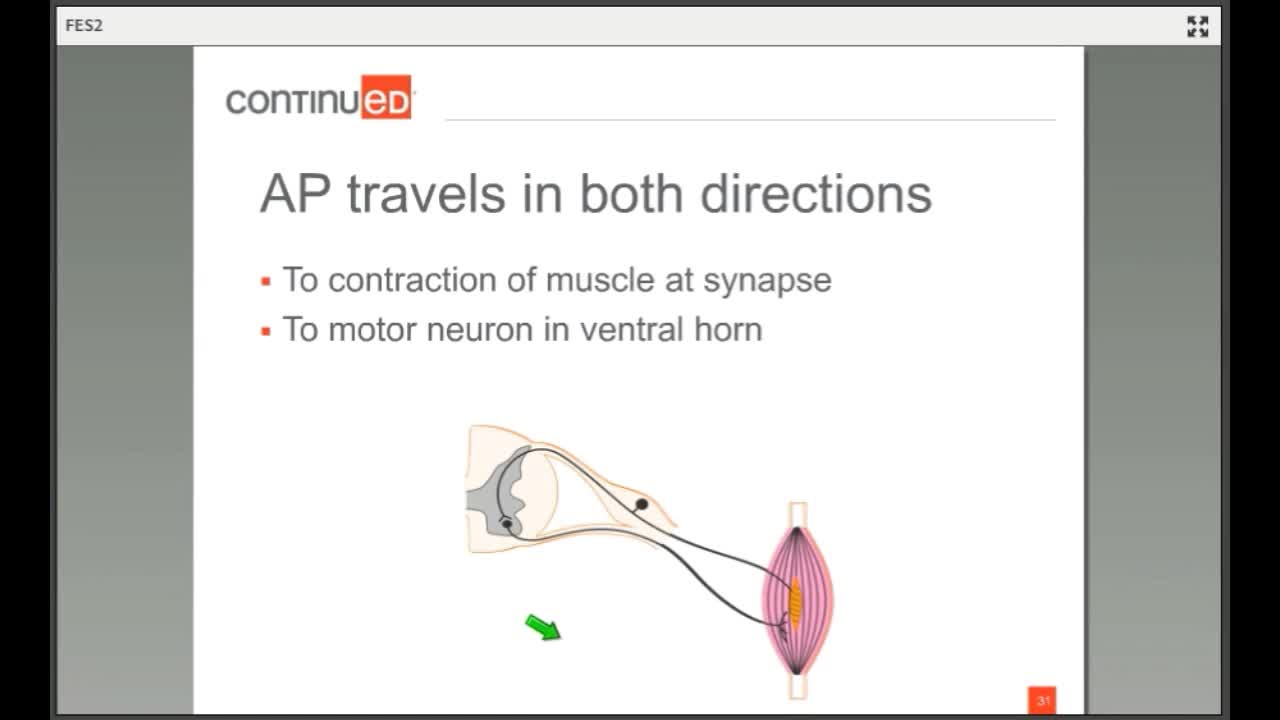
This is your lower motor neuron or the nerve that originates in the anterior horn of the spinal cord and travels out to the muscle. We simulate this nerve to make this muscle contract. The information gets picked up by end organelles and sensory fibers saying, "We did it," goes back in through the dorsal root of the spinal cord.
Action Potential Travels in Two Directions
- To the contraction of the muscle at the synapse
- To the motor neuron in the ventral horn
There are two important distinctions. The first is that the action potential travels in both directions. In a physiologically generated contraction, there is a wave of hyper-polarization behind it preventing a retrograde impulse. When we stimulate here, there is not that wave of hyper-polarization behind it, so the stimulus goes this way and this way. The stimulus goes to the motor neuron in the ventral horn and to the muscle synapse.
All or Nothing Contractions
- Electrically elicited contractions lack smooth, gradual onset of voluntary contraction, reflecting biased and synchronous motor unit recruitment.
- Voluntary contractions allow for asynchronous activation of varied motor units which allows for smooth switching between active and inactive motor units to maintain muscle activity while allowing recovery time for individual motor units.
The second big difference is that electrically driven contractions lack smooth gradual onset like a voluntary contraction reflecting a biased and synchronous motor unit recruitment. The body is smart so when stimulation is delivered, it is going to capture whatever motor units are closest. Those are the ones that are going to become stimulated and contract. Whereas, in a voluntary contraction there is asynchronous activation of varied motor units necessary only for the specific task. If I go to pick up a bottle of water, my brain knows roughly how big it is, how much power is needed to close my hand, how heavy it is, and how much to contract to maintain that grip. In contrast, the electrically driven contraction does not know that it is not smart enough to know that. It is going to just stimulate all the fibers and you are going to get this all or nothing contraction.
Recruitment of Motor Units
- Axons of the largest diameter are the easiest to activate and are recruited before axons of smaller diameter.
- Recruitment of motor units by electrical stimulation progresses from large to small, the reverse order of voluntary contractions.
- Recruitment is also a function of electrode proximity
The recruitment of motor units is different in an electrically driven contraction. Axons at the largest diameters (fast fatigue fibers) are recruited firs as they are more easily stimulated. Whereas, in a voluntary contraction, you are only going to use what you need and you tend to start with the smaller fatigue-resistant fibers.
Fatigue Occurs More Rapidly
- A greater proportion of fatigable motor units is necessary for a given contraction.
- Combining voluntary contractions with ES produces the best and strongest contraction, as the ES recruits different motor units not activated at a given moment by a voluntary contraction.
Fatigue occurs more rapidly because of a greater proportion of fatigable motor units needed for a given contraction with electrical stimulation. It is also why we encourage our patients to try to use voluntary effort along with the stimulation to generate as smooth of a graded contraction as possible that includes both fast fatigue and fatigue resistant fibers.
Stimulation Current and Parameters
When it comes to stimulation current and parameters, we now have the capacity to manipulate the stimulation to drive the desired response.
Electrodes and Placement
- Use as small of an electrode as possible
- One that will recruit entire muscle
- Minimizes fatigue and bleed
- A larger electrode will be more comfortable
- Electrodes should encompass motor point of targeted muscle
- Use the largest cross-sectional area
- Consider skin health, factors of impedance
The first thing we can do is choose where we are putting our electrodes. We want to use as small an electorate as possible, but one that will recruit to the entire muscle. We want to minimize fatigue and "bleed". When we talk about bleed, we are talking electrical bleed with the electricity spreading into muscles that you do not want. Obviously, if you are stimulating the small muscles of the hand, you are not going to use the same size electrode as you would if you were stimulating the bicep or the shoulder. However, a larger electorate will be more comfortable because it spreads out the current over a larger area versus concentrating it into one spot. You want the electrodes to encompass the motor point of the targeted muscle, which is theoretically the largest cross-sectional area. You need to consider factors like skin health and factors of impedance. If you are stimulating over an area that is very hairy, a lot of callouses, or a lot of adipose tissue, it will be harder to conduct that electricity. In these cases, you might need to move your electrode or change your parameters.
Alterable Parameters
- Waveform: biphasic (symmetrical or asymmetrical), monophasic
- Frequency: hertz (pulses per second)
- Amplitude: milliamperes
- Ramp (surging): time to a maximum amplitude
- Duration: total treatment time and individual pulse (microseconds)
There are five parameters that are typically controllable on any given stimulator. The kind of stimulator you are using though will depend on how much control you have.
Waveform
- Monophasic
- One phase each pulse
- Also known as pulsating DC
- Unidirectional flow
- One electrode is positive and the other is negative
- Biphasic
- 2 opposing phases are contained in a single pulse
- Asymmetric and Symmetric
- Symmetric is preferred to asymmetric if motor neurons are the target
Let's first look at the waveform. Generally, stimulators have either a monophasic or a biphasic stimulation, although the monophasic is being phased out because it has very specific applications. A monophasic wave has only one phase in each pulse, whereas a biphasic waveform has two opposing phases within a single pulse. A monophasic is also known as pulsating direct-current, and biphasic is alternating current because you have a unidirectional flow of electrons. There is also a risk of burns with monophasic stimulation.
Here is what those look like in Figure 11.
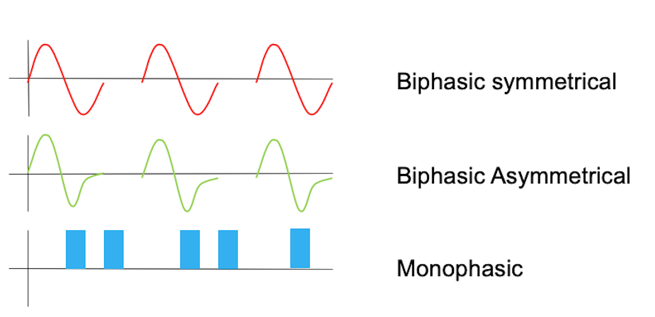
Figure 11. Examples of waveforms.
This is a biphasic waveform, right, so it has a single pulse, it has both a positive and a negative charge. This one is symmetrical, whereas this one is asymmetrical, there is, so the positive charge is greater here than the negative charge. The monophasic waveform, again, has the charge in only one direction. So that the pulse is uneven, the current is uneven and you get an increased risk for burns in that way.
Amplitude
- The magnitude of a current (or voltage)
- Peak amplitude: maximum current during a phase
- Measured in milliamps (mA)
The amplitude is the magnitude of the current or the voltage. If we look at Figure 11, the amplitude is the height of the curve. In a second, we are going to talk about duration which is the total length of the curve. I hope this helps you to visualize the stimulation in this way, and then you can think about how you are manipulating it overtime.
Frequency
- The number of pulses (waveforms) repeated at regular intervals
- Referred to as pulses per second (pps) or Hertz (Hz)
- The inverse relationship between pulse frequency and tissue resistance
The frequency is the number of pulses repeated at regular intervals and is measured in pulses per second or Hertz. There is an inverse relationship between pulse frequency and tissue resistance. So, if you have a tissue with a lot of resistance, you might do better with a decrease in frequency.
Pulse Duration
- The total time elapsed from the beginning to the end of one pulse
- Includes the phase duration of all phases and the interphase interval
The pulse duration is the total time elapsed from the beginning to the end of one pulse and includes the phase duration and the interphase interval.
Minimize Current
- The primary goal is to get motor action or neurological benefit with as little external input as necessary while minimizing fatigue.
Your goal as the therapist is to get a motor action or neurological benefit with as little external input as necessary while minimizing fatigue. I hope I convinced you early on that it is important to do a lot of repetitions. This high-volume, high repetition training is beneficial to your patients. However, if you are stimulating them with these fast fatigable fibers, you are going to wind up having poor contractions as time goes on. Manipulating those parameters will help you to prevent that. This is also why the TENS or pain modulating stimulation is not ideal because that stimulates at a high frequency, and frequency is the primary contributor to fatigue.
Parameters
- Sensory (TENS)
- High frequency (80-100 Hz)
- Low pulse duration (80-100 μsec)
- Amplitude sub-motor
- Motor (NMES, FES)
- Low frequency (20-60 Hz)
- Longer pulse duration (100 μsec-1 millisec)
- Amplitude to tolerance
Sensory level settings are generally high-frequency and low pulse duration at a sub-motor amplitude. Motor stimulation is low-frequency, somewhere between 20 and 60 Hz with longer pulse duration, and the amplitude here is to tolerance. For those of you who have stimulators where you can adjust parameters, I hope these words are all familiar to you.
Starting Parameters
- Ex: 20 Hz, 200 μsec, timing based on condition/activity
- Pick a place to start. Adjust based on what you see.
- Can the pt. tolerate it?
- Can you reach tetany?
- Are you getting a capture of the whole muscle?
- Are you getting the action you wanted?
- Are you getting bleed into other muscles?
Start here and then adjust based on what you see. The parameter of 20 Hz and 200 microseconds will be right for more than half of the muscles that you need to stimulate. Then, you are going to adjust based on what you see. Can the patient tolerate it? Did you reach tetany? Are you getting a "capture" of the whole muscle and the action you wanted? You do not want to get bleed through into other muscles.
Alternating Parameters
To that end, my therapists and I have developed this chart in Figure 12.
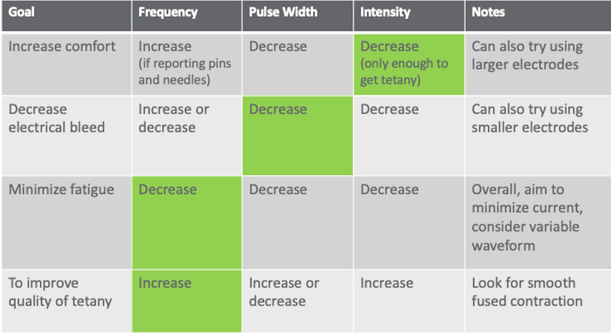
Figure 12. Electrical stimulation parameters.
It is in your reference list. If your patient is complaining of pain, the first thing you would do is decrease intensity. When you decrease intensity though, you might lose your tetany. Tetany is that smooth muscle contraction that gets you through most of the range that you are after or that the patient has available.
The other problem is once you start to change one thing, you are going to have to change another thing. It is like an "if you give a mouse a cookie moment." If you decrease your intensity and you lose tetany, you are going to have to make a change somewhere else. I tell my therapists to make only one change at a time. Start in the green box, and then do what you need to do to get the other thing. In another example, if you lose your tetany because of decreased intensity, then you would go back and increase your frequency. Bleeding through into other muscles is really common especially if you are trying to stimulate the triceps, for example as this tends to be a pretty spindly muscle on most people with neurological injury. You might get a lot of bicep pull-through instead. To correct this, you would decrease your pulse width first. Then, if you notice that the patient is fatiguing really quickly, meaning that you are getting maybe only one or two contractions through the full range, and then the subsequent contractions are less and less, you can try decreasing your frequency. Decreasing your frequency will help to protect against fatigue because less of the fast fatigable fibers are being contracted repeatedly. And then, if you want to improve the quality of the tetany, as I mentioned before, you would increase the frequency. You would need to reach that temporal summation feature in order to get smoothing out of the muscle contraction. I find this to be a really handy chart. When you start working for me in our clinic, I hand it to you and you put on your clipboard. Over time you start to internalize these procedures. I encourage you to do the same if you are just starting out with electrical stimulation.
Designing Treatment
Now, let's look at our patient.
Goal
- Maximizing Efficiency
- To avoid fatigue and accommodation, use low frequency and gradual increases in intensity
Remember, our goal is to maximize the efficiency of the delivery of your stimulation and to avoid fatigue and accommodation.
Assessment
- Evaluate muscle and nerve health to determine the most appropriate intervention strategy.
- Must have an intact peripheral nerve to stimulate.
- A stimulable muscle must have some intact LMN
- Look for PN injury with ortho conditions
- Look at the pattern of damage to CNS with neuro conditions
The first thing we need to do is an assessment. We need to evaluate muscle and nerve health to determine the most appropriate intervention strategy. Again, we must have an intact peripheral nerve to stimulate. The great news for things like stroke, brain injury, and CP is that these are upper motor neuron conditions and are very easy to stimulate. Spinal cord injuries tend to be lower motor neuron issues, like with brachial plexus injuries, which can be very difficult to stimulate. The good news here is that most muscles have more than one level of intervention, and so depending on what the injury is, you might be able to capitalize on some of the redundancy built into the nervous system. Peripheral nerve injuries associated with orthopedic conditions might also present problems, but those are generally peripheral nerve axon injuries and will usually regrow. Stimulation can be useful at that motor endplate to direct that axonal regrowth.
Atrophy and Muscle Health
- Long-term atrophy may result in muscles that are too weak to move against gravity, even with electrical stimulation.
- Spindly motor units are sluggish to respond to electrical stimulation.
- Additional considerations:
- Connective tissue and skin health
- Hydration and nutrition
Secondly, we need to look at atrophy and muscle health. Long-term atrophy may result in muscles that are too weak to move against gravity even with electrical stimulation. Also, spindly motor units can be sluggish. In order to give these motor units the best possible chance to respond, especially if they are slow and skinny, we want to stimulate in a slow way with a decreased frequency for more extended periods of time. This is an inverse relationship. The fast fatigue fibers are going to be stimulated first so we need stimulation for a longer period of time. Again, low-frequency, long pulse durations are required for these patients.
UMN vs. LMN: Complicating Factors
- Patterns of innervation:
- More than one segment innervates each muscle; it will not be clear what myotome or peripheral nerve distribution.
- Long-term atrophy can appear as LMN injury secondary to weakness
- Try extending pulse duration to reach slow-twitch fibers
- Not a one-time trial. Treat for 6-8 weeks with careful dosing before making a determination.
- The patient will still receive secondary benefits: sensory stimulation, neural activity, peripheral, vascular, etc.
Of course, I make it sound easy. If it were easy to check for reflexes to determine upper versus lower motor neuron issues, my life would be much easier. What we generally do is try extending the pulse duration to reach the slow-twitch fibers, as I mentioned, and then we treat for six to eight weeks before we make a determination that a patient is not responsive. I have had some of my neurologist friends do things like a needle EMG to figure out what is truly upper versus lower motor neurons. Honestly, it changes over time and depends on where they put the needle. At that moment, there may not be any fibers that you can rescue with electrical stimulation, but over a six to eight week period, you might see some benefit. There are also secondary benefits of electrical stimulation like sensory stimulation, activity into the nervous system, and increased peripheral and vascular health.
Spasticity
- An increase in muscle tone due to hyperexcitability of the stretch reflex and is characterized by a velocity-dependent increase in tonic stretch reflexes
- UMN Syndrome: Lack of input from corticospinal tracts
Spasticity is muscle hyperreactivity. There is this hyper-excitability of the stretch reflex, and it is associated with a lack of input from corticospinal tracts as is seen in upper motor neuron syndromes.
Impact of Spasticity on Therapy
- Unable to access normal movement patterns
- Masks underlying activation or strength
- Decreases ability to participate in mobility activities
- Safety impact
Spasticity can be really problematic for patients because they are unable to access normal movement patterns. It can also mask underlying activation or strength, decrease a person's ability to participate in mobility activities, and may impact safety for some patients who get thrown into positions that can cause wounds or pressure ulcers.
Spasticity Management
- E-stim also helps to strengthen muscles and has analgesic properties which both help to decrease spasticity.
- Successful clinical applications include long ramp times, to minimize stretch reflex, and variable pulse frequency and duration, to reduce summation and accommodation.
We can use electrical stimulation to strengthen muscles and provide an analgesic or pain-reducing property which both help to decrease spasticity. Successful clinical applications for spasticity include long ramp times to minimize the stretch reflex and variable pulse frequency and duration to reduce summation and accommodation.
E-Stim for Spasticity Management
1. Sensory settings to spastic muscle
- Applied prior to the activity
- Applied during the activity
- Examples: Sensory e-stim to quadriceps either in the car on the way to therapy, or during gait, to increase knee flexion during swing
2. Motor settings to spastic muscle
- Applied prior to activity (fatigue)
- Example: Motor e-stim to quadriceps while standing in the standing frame to fatigue quads, done prior to gait training, to increase knee flexion during swing
3. Motor settings to the antagonist
- Applied during activity
- Example: Motor e-stim to hamstrings during gait training to increase knee flexion during swing
You have three choices when applying e-stim for spasticity management. The first is using a sensory setting. This is a high-frequency, low pulse duration to the spastic muscle. This can be applied prior to or during the activity. For example, we might prescribe e-stim to the quadriceps in the car on the way to therapy or during gait to increase knee flexion during swing. We can use motor settings to a spastic muscle prior to the activity. This produces an immediate reduction in the appearance of spasticity because of fatigue, but it also helps to modulate firing sequences by enacting the interneurons between the dorsal and anterior horns of the spinal cord. Or, you can apply motor settings to the antagonists. For example, you might stimulate both a client's spastic bicep and the tricep during a reaching activity or during some self-care practice.
Case Example
Here is a case example of gain training. As a disclaimer, I am an OT who studies walking.
Video #3.
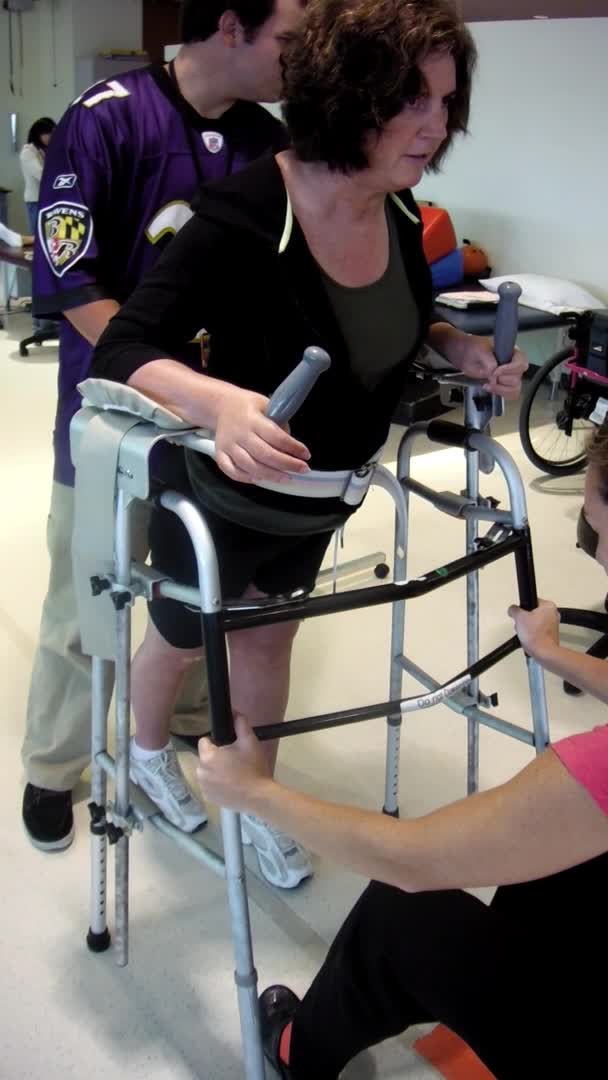
This is a patient before a bout of electrical stimulation. Her gait is really stiff, and she is having to initiate walking by using her head and trunk to get her leg through. You can see that she is hyper-extending in both knees during the stance phase and her steps are really short. She has sort of a step to instead of a step-through, particularly on the left side. See how that right knee snaps back into hyperextension when she steps.
Video #4.
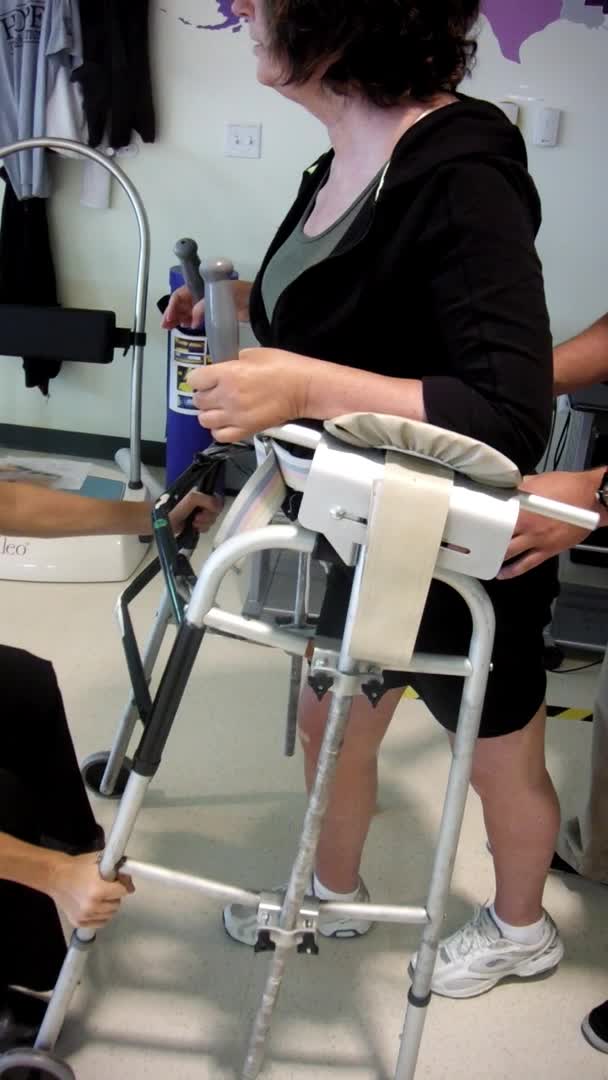
Here she is after 30 minutes of FES cycling, which is a repeated stimulation scenario. You can see she has more control during that stance phase. And, she does not immediately snap back into that hyperextension in her right knee. She still gets there, but it is not quite as quick or as violent. Her steps are a lot longer and her upper body is much quieter. She is also not having to initiate with her head and trunk as she did before. I hope you can see that difference. This was only after 30 minutes. Can you imagine if we stimulated for longer? With that short training, she demonstrates a more normal kinematic gait pattern. We were able to strengthen muscles that are antagonists to the ones with spasticity and improve her overall gait pattern.
Special Patient Populations
Spinal Cord Injury (SCI)
- Spinal stability
- ES to LE while in traction/halo to provide input without motion
- ES to LE to lower risk for DVT
- Medical fragility
- ES to LE and trunk before getting out of bed or during upright to offset orthostasis
- Bedrest
- ES to dorsiflexors or wrist extensors to offset ortho complications
- In ICU: ES early and often!
Needham, Truong, Fan, 2009
Obviously, I have the spinal cord injury bias because that is where most of my work is. We stimulate all the time. As soon as somebody is stable, we start stimulating in acute care. We will stimulate lower extremities while they are in traction or in a halo to provide input without motion. We can also use stimulation to the lower extremities to lower the risk for a DVT. This might also be good for some of your nursing home patients. As soon as they are ready, we can use electrical stimulation to help with their medical status, helping them get out of bed, or during upright positions to offset orthostasis. We can also use it during bed rest to prevent contractures or orthopedic complications. And at John Hopkins, they have started stimulating in the ICU, used this to facilitate early mobilization, and have shown shorter ICU stays and better outcomes.
- Retrospective cohort to examine the effect of long-term lower extremity FES cycling on the physical integrity and functional recovery in people with chronic SCI.
- FES during cycling in chronic SCI may provide substantial physical integrity benefits, including enhanced neurological and functional performance, increased muscle size and force-generation potential, reduced spasticity, and improved quality of life.
Sadowsky et al., 2013
There is some evidence about the effect of FES specific to cycling on muscle perfusion and muscle size and composition in response to stimulation. Muscle size and composition also lead to improved force-generation potential, reduced spasticity, and improved quality of life.
Multiple Sclerosis (MS)
- Several studies involving ES for foot drop:
- Decreased effort in walking
- Increased walking speed
- Improved stair negotiation, increased ankle DF
- Increased quality of life
Taylor et al., 1999; Sheffler et al., 2009
There is this thought that we should not exercise patients with MS. However, we have learned over the last 10 or 15 years is that this is not actually true. There are several studies involving electrical stimulation for foot drop in MS that show a decreased effort in walking and improvement in walking speed, specifically stair negotiation, ankle dorsiflexion, and improvements in quality of life.
- Pilot study:
- 5 patients with primary or secondary progressive MS
- Cycled in the home for 6 months
- Results:
- Improvements in 2-Minute Walk Test, Timed 25-Foot Walk, and TUG
- Strength improved in muscles stimulated by FES cycle
- Multiple Sclerosis Functional Composite (MSFC) and physical and mental health sub-scores and total SF-36 improved
- Conclusions: FES cycling was reasonably well tolerated by progressive MS patients
Ratchford et al. (2010). A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. Neurorehabilitation, 27(2), 121-8.
Again, specific to functional electrical stimulation cycling, there is a study in MS where they looked at patients with both primary and secondary progressive MS who did an FES cycling program. They not only did they improve their walking capacity and strength, but they saw an up-regulation in all the neuroprotective factors like BDNF and in the down-regulation of all the inflammatory markers that are typically associated with MS.
Cerebral Vascular Accident (CVA)
- Cognitive limitation
- Ensure pt has a way to express discomfort
- ES improves uptake of Botox
- ES as a compliment to splint program
Again, stroke is also a great target population for electrical stimulation. The only thing here is that you want to make sure that they have a way to express discomfort if there is any aphasia or cognitive limitation. There are studies to show that electrical stimulation improves the uptake of Botox and is a really nice compliment to a splinting program when addressing contractures.
Cardiac
- ES increases cardiovascular demand
- Pacemakers v. Defibrillator
- Depends on type
- Location of stimulation
Crevenna, et al., 2003
You want to be very careful with this population especially around their pacemakers and defibrillators. I had a patient who had essentially an internal decapitation with a very high-level spinal cord injury. He was on a ventilator. He had a diaphragmatic pacemaker, and then because he was elderly, he had some pre-existing cardiac conditions and wound up with a defibrillator. The family was so adamant about continuing their FES program that they convinced Medtronic to come in and monitor the defibrillator during stimulation. And surprisingly, it did not interact. I am not suggesting that you do this, but it is good information for you to know. If you have a similar situation, I would reach out to the device representatives to help you.
Orthopedic
- No ES close to the external fixator
- OK around internal hardware
- Respect surgical precautions
- ES offset atrophy associated with immobilization
- TENS
- Sensitive skin electrodes for fragile or irritable skin
In orthopedic populations, you want to stay away from the external fixator because that is metal and will conduct electricity, but it is okay around internal hardware which is typically titanium and does not conduct electricity. Plus, internal hardware becomes encapsulated in scar tissue over time which also will not conduct electricity. You need to respect surgical precautions just like you would in any other situation. You can also use e-stim to offset atrophy associated with immobilization. I have had patients with casts where we have cut windows into their cast so they could continue to have electrical stimulation. There are also skin electrodes for fragile or sensitive skin.
Pediatric
- Start slow
- Decorate electrodes
- Wear electrodes only
- Demo on Mom/Dad
- Minimize current
- Can cut down the electrode
- Distraction!
Kids can be tricky, but they will surprise you. Kids tolerate electrical stimulation remarkably well. We generally tend to start really slow or put on electrodes without plugging them in at first. I have decorated electrodes to make them more fun and have also demonstrated on mom and dad. One of my favorite tricks is to give the patient the trigger. This way they know that they are in control. And then once you get them engaged in an activity, they tend to forget about that.
Functional Application
To Augment Voluntary Effort
In this video, I have a patient with a C5 injury who has no hand function. They have good shoulder and elbow function. I am stimulating him to pinch and then lift the card.
Video #5.

You can see I have electrodes on the wrist and finger flexors, and one on the thumb adductor. You will see the finger flexors come on there, and then, he goes into a little bit more flexion. Then, he has the thumb adduction on top of that to get a lateral pinch.
To Improve Kinematics and Endurance
In this video, you will see that this patient is working in the Saebo mobile arm support, and she is doing a vertical reaching task.
Video #6.

She is playing a game on the iPad, but it requires her to reach up. You can see that she does not have much tricep function and a lot of weakness in her shoulder. She also leans to the right to compensate in order to reach all the way to the top of the screen. She then has to use her head to reset and shrugs to activate her upper tracts instead of her anterior deltoid for that shoulder elevation. This is her baseline.
Video #7.
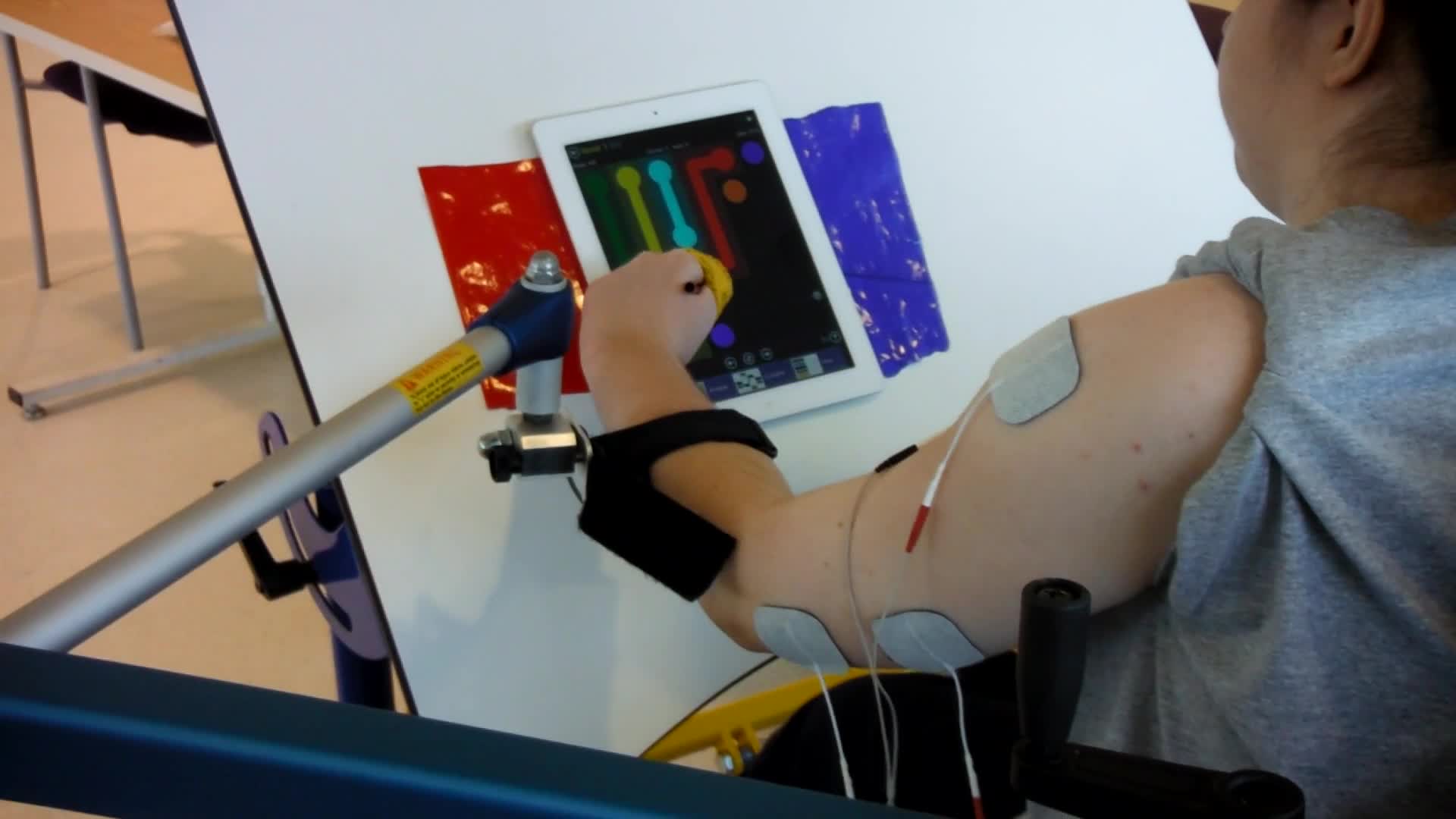
In this video, we have added e-stim to her anterior deltoid, her middle deltoid, and her tricep. Now, when she starts to reach, she stays more neutral. She elevates from her anterior deltoid and less from the upper tract. You can see she seems much more stable in her reach with the firing of her tricep. She also does not have to compensate with her trunk.
Here is an example of another client.
Video #8.
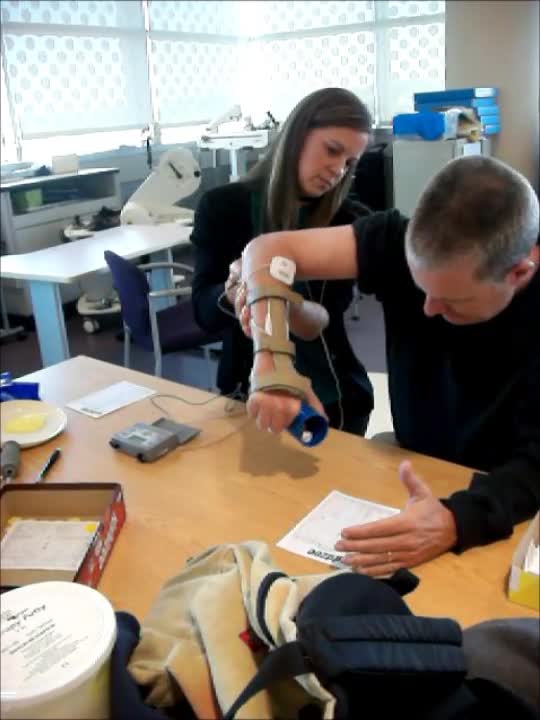
Due to a stroke, he has a lot of upper extremity spasticity. In this video, the therapist has triggered stimulation on his wrist extensors and finger extensors. He is wearing the brace on top of it to help eliminate some ancillary wrist motion and really get some finger-opening. As the therapist pushes down the trigger, he starts to get some opening. He is slow to respond because he has so much spasticity in his flexors, and the therapist has to help him so that he can get the cup in his hand.
Video #9.

In the video, we adding sensory level stimulation to his bicep and wrist flexors with the same triggered stimulation to his extensors. As you see, his hand opens much easier. Now that we have already started to relax the bicep and the wrist flexors when we add the motor stimulation to the antagonist, he opens much more easily to be able to get that utensil.
FES to Paraspinals During Seated Play
The only limit in using stimulation is your own creativity. Wherever you feel like the patient is not activating on their own is where you can add it. Figure 13 is an example of a little kid who has this kyphosis because her injury is a bit higher. You can see that we provide stimulation all down her back to help her with some sitting taller.
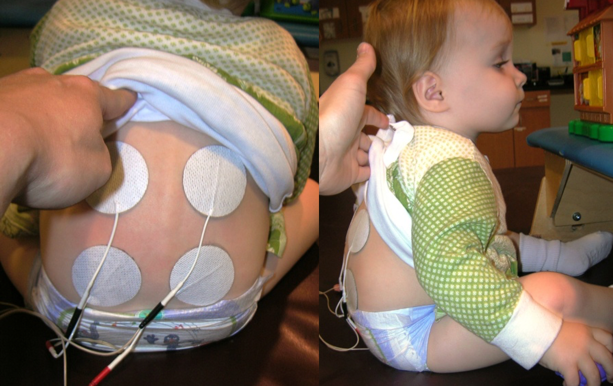
Figure 13. E-stim to paraspinals.
FES to Gluteals During Half Kneeling
Here we have used FES to the glutes in a half kneeling position to help with some stability. (Figure 14).

Figure 14. E-stim to glutes in a half-kneel.
FES to Hip Flexors Using Trigger Switch During Crawling
Here we are using alternating hip flexor
