Introduction
Today, we are going to be talking about neuromodulation for spinal cord injury. This is a hot topic in today's research for spinal cord injury. My current clinical research centers on this, but it does tend to be focused heavily on walking. I am an OT that studies walking, but what I am going to convince you today is:
1. As OTs, we should care about walking.
2. There is a role for upper extremity recovery in relation to neuromodulation.
Spinal Cord After Injury
This is a spinal cord injury in Figure 1.
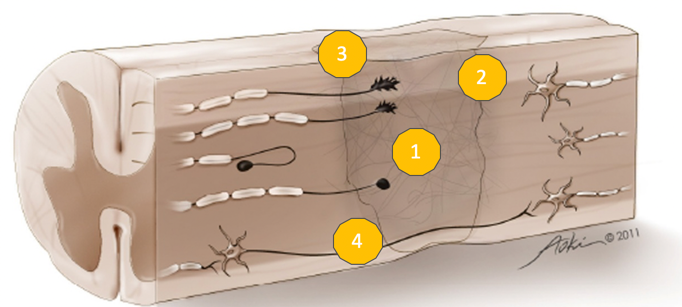
Figure 1. Spinal cord injury. Image used with permission.
This is your spinal cord after injury. We see that there is an initial loss of cells. That initial mechanical trauma causes secondary cell death or apoptosis which results in additional cell loss and cavitation. That cavitation eventually fills in with fluid and forms a cyst, which is the first barrier to recovery. Second is the glial scar that surrounds the cyst. This scar is thick and fibrous and represents a mechanical barrier to recovery. It is also a chemical barrier to recovery as it releases inhibitory phytochemicals letting the spinal cord and surrounding tissues know that this is a zone of damage and you should stay away. Additionally, axons can be transected.
In Figure 2, you can see an axon has entered the zone of damage, but it cannot get through it because it is transected, and there are some axons that are demyelinated.
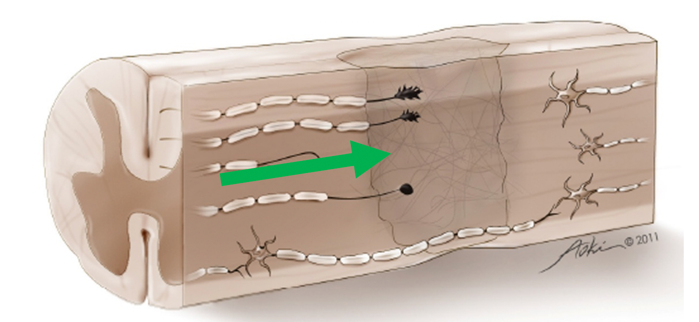
Figure 2. Axon trying to enter the zone of damage. Image used with permission.
In both cases, this represents a conduction block. Those axons fail to carry information from the brain to the muscle. For natural recovery, how do we get the axons to grow through the zone of damage and synapse onto axons on the other side? There has been lots of work done to look at ways to bypass the zone of damage with scaffolding or peripheral nerve bridges that might even bypass the zone of damage by going around.
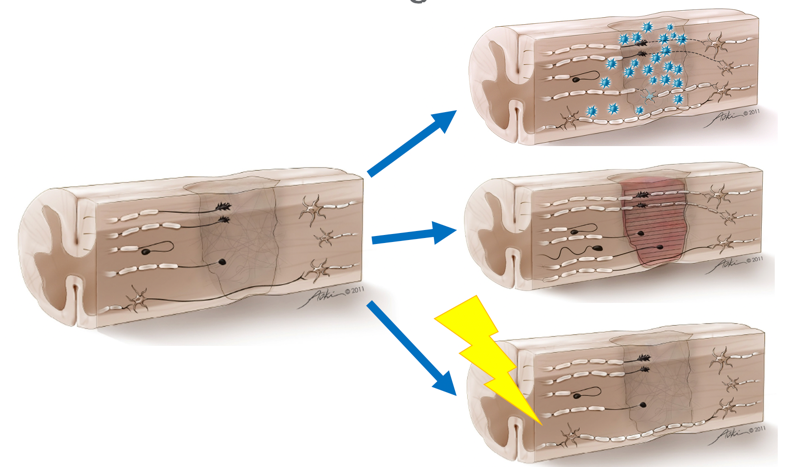
Figure 3. Ways to bridge the zone of damage. Images used with permission.
Scaffolding would go through to direct axonal regrowth (top picture). There has been a lot of research done with growth or neurotrophic factors to encourage these buds to continue to straighten, move through the damaged zone, and synapse on the other side. Also, there is work being done on remyelination as that seems like it might be a more near-term solution. In SCI, about 10% or more of your spinal cord remains in most cases unless you are talking about penetrating trauma. There is the potential that we can reorganize and use what is still intact. As I mentioned, we are looking at putting things into the central nervous system by administering exogenous cells, like stem cells or growth factors directing axonal regrowth to build scaffolding or a bridge through the zone of damage. Finally, the thing that as therapists we have control over is supercharging the synapses and connections (bottom image) that are still intact. This is our role. How do we help these axons to carry information across the damaged zone and do it efficiently?
Combination Strategy
- Neuroprotection
- Bridging
- Growth factors
- Transplantation
- Rehabilitation
Many scientists like a combination strategy. Real recovery is going to require some neuroprotection or protection and stopping that cascade of secondary events causing apoptosis. There also has to be bridging or scaffolding to bypass the zone of damage and growth factors to direct it. There is a lot of research on stem cells and transplantation, but everybody agrees that rehabilitation is going one of the most important factors. None of the other things are going to matter if rehab is not in place. I remind my patients of that fact. "If you let your body decompose by letting muscles shorten and bones and connections weaken, then you will not be ready for whatever magic pill they invest in the next five years or whatever it is going to be."
Activity-based Therapy
Activity-based therapy is sort of the original form of neuromodulation in terms of increasing a lot of input into the nervous system to drive the desired output.
Paradigm Shift
- Compensation►Restoration
- Scientific evidence of activity-dependent plasticity in CNS
- Development and acceptance of rehabilitation interventions aimed at restoration (FES, LT)
- Patients pushing previously established limits and expectations.
- Specific environments/equipment can be limiting
- As demographic rehab skews younger, push for community integration
There has been a shift in rehabilitation research. We are moving away from traditional models of compensation towards models that encourage restoration of function. I always get a little heat from OTs about this. In the past, we looked at how to modify the person, the environment, the occupation, or whatever it is. We want people to participate more fully, and I am on board. I want that as well. However, if I can help to restore grasp in a patient, they can do work tasks and get dressed. Whereas, if I only give them a built-up utensil and a lip to plate, they can only feed themselves. We need to think about what kind of low-level skill they can be independent in so that they can then generalize that skill to lots of different activities or occupations. I really push OTs to think about what we are offering patients as different. How many of you have had the experience where you have worked with a patient to get them independent with adaptive equipment or modifications, and then they go home and they do not get it quite right. Or, they lose or break the equipment. Many times they do not want to go out to dinner with a bag full of their own utensils. They start to abandon those things, and it just becomes easier for somebody else to help them. On the other hand, had you spent a little bit more time helping them to restore these component-level skills, they would have generalized those to many activities and potentially gotten better over time. In a minute, I am going to show you some evidence for this concept.
This paradigm shift where we are moving from compensation to restoration is based on the science that shows that there is plasticity in the nervous system, in both the brain and the spinal cord. However, this plasticity is activity-dependent. You have to use it or you will lose it. We have to provide input below the level of the lesion or around the brain lesion in order to see that recovery. Fortunately, we are in a time where there has been this large development and acceptance of different rehabilitation interventions aimed at restoration. Functional electrical stimulation and locomotor training are my two favorites, and if you missed my talk yesterday, I spent a whole hour talking about electrical stimulation. Patients want to have fewer limits, and they have greater expectations for their recovery than they did 10, 15, or 20 years ago. Due to specific environments and types of equipment, this can be very limiting for patients. Then, as the demographic in rehab starts to skew younger, we are seeing less time in rehab for things like strokes and even spinal cord injuries, and we are seeing more catastrophic injuries in rehab in younger kids and younger adults. And, they need to be able to reintegrate into the community.
Repetitions in Traditional Rehab
This is a study that came out in 2009 from the University of or Washington University in St. Louis where they looked at 312 therapy sessions in post-stroke rehab.

Figure 4. Not enough repetitions (Lang et al., 2009).
How many minutes of therapy do you think you are actually giving patients? If you just want to use your chat box to write down a number. The numbers coming in are 30, 40, 45, and 50. Yeah, you guys are a smart group. The real number is 36. I get it as I have worked in inpatient rehab. Somebody has to go to the bathroom. They need their medication. You have to put on a splint before you can get the client to therapy. You can see how this happens. Now, again, in your chatbox, if the goal of the session is upper extremity functional movements, how many repetitions do you think they are doing in those 36 minutes? The answers are 20 to 30. The answer is 32 repetitions. The truth is that we are doing less than one minute when you factor it out over your session, and that is not enough. It gets worse if we are talking about lower extremity movements, only six during gait and transfers.
- “Amount of practice…is small compared with animal models…Current doses…during rehabilitation are not adequate to drive neural reorganization needed to promote function post-stroke optimally.”
The amount of practice is small compared with animal doses. Current doses during rehabilitation are not adequate to drive neuronal reorganization needed to promote function post-stroke optimally. I am sure people have seen the videos of rats walking on a treadmill. I mean those rats are walking on that treadmill three hours a day in some cases or even more (six or eight) and on top of that, we are not giving those rats wheelchairs and adaptive utensils. They have to figure out how to get around and feed themselves, or they die. Now, I am not suggesting that we do not give people wheelchairs and adaptive utensils, but I am suggesting that we expect more out of our patients, and that is where activity-based therapy comes in.
What is Activity-based Therapy?
- Repeated near-normal activity, above and below the level of the lesion, intended to:
- Optimize the neurological system
- Offset the rapid aging, physical deterioration and secondary complications associated with SCI
- Characterized by:
- High-intensity practice
- Task-specific and patterned activity above and below the level of the lesion
- Goal:
- Restore CNS function
- Promote neural recovery and regeneration
Anybody who spends any time with me on any given day will hear me say this at least once. Activity-based therapy is repeated near-normal activity above and below the level of the lesion to optimize the nervous system for recovery and offset the rapid aging, physical deterioration, and secondary complications associated with spinal cord injury. This is it. This is the game. How do I do more and how do I do it closer to normal? We use high-intensity practice at both task-specific and patterned activity above and below the level of the lesion to restore central nervous system function and promote neural recovery and regeneration. This is how we help our patients actually get better.
Key Components of ABRT (Activity-based Restorative Therapy)
Here are the 5 key components. This helps us to structure sessions and to decide what patients need in the moment and phase of their recovery.
5.Task-Specific Practice
Functional Electrical Stimulation
This is an example of a functional electrical stimulation bike.
Video 1.
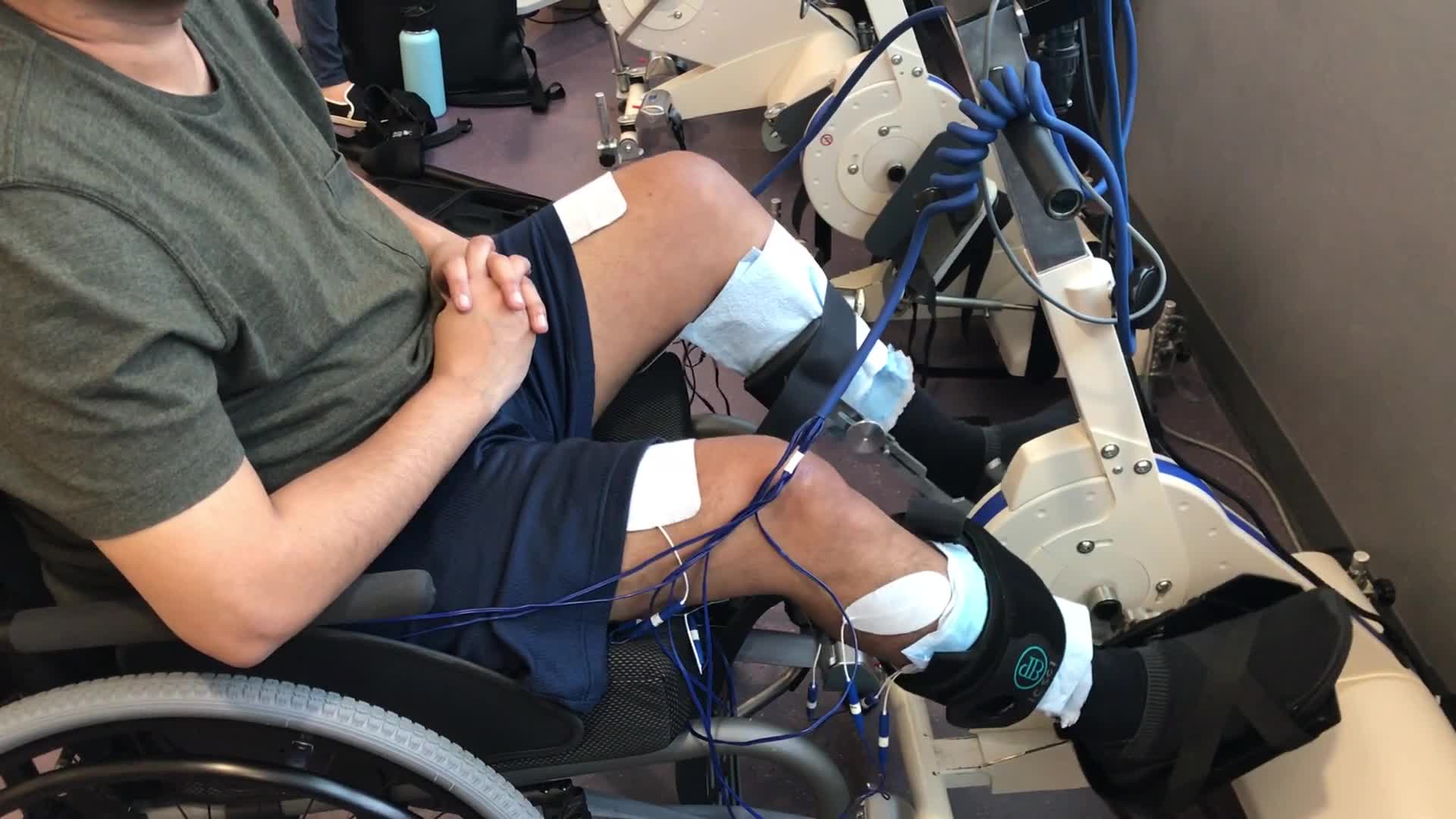
This patient is wearing electrodes over their skin to use stimulated muscle contractions in order to make the cycle move. As I mentioned about FES (I gave a full lecture on that yesterday), it is a way for us to get a lot of input into the system efficiently. We are stimulating those nerves that the patient would not be able to do otherwise. It has all of these secondary health benefits too: muscle size, composition, perfusion, bone health, and cardiovascular health. It can also help to normalize tone and optimize the nervous system for recovery. In fact, all of our interventions in activity-based therapy help to supercharge the nervous system to take advantage of the connections and have secondary health benefits so we can make sure to maintain the body and its integrity.
Weight Bearing
This is an example of weight-bearing.
Video 2.
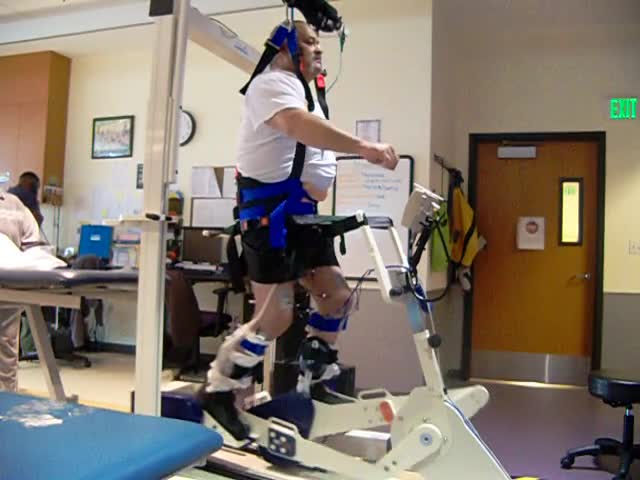
Next, we have taken the same FES and put the patient in an upright position. Now, the stimulated contractions are helping him to bear weight through this elliptical-type motion. Weight-bearing is something that you are probably incorporating into your treatment now. We can weight bear through arms and hands as part of a quadruped position or a posterior prop position. You might even be doing some of your interventions in standing. How would it change for a patient if they could do their toothbrushing standing at the sink instead of sitting at the sink? It would be closer to normal and a less restrictive environment.
Benefits of weight bearing:
- Improved bowel and bladder function
- Decreased number of bedsores
- Improved range of motion
- Improved autonomic regulation
- Decreased spasticity
- Improved bone mineral density
- Improved cardiovascular function
- Improved motor function
Improved quality of life
Weight-bearing also has additional health benefits in bowel and bladder function, decreased number of bedsores, improved range of motion, autonomic regulation, spasticity management, bone health, cardiovascular health, motor function, and overall improved quality of life. When I first started in spinal cord injury, I thought, "Even if patients do not move, if I can make them feel better at engaging the environment more successfully as I'm improving their quality of life." But it turns out, if I actually make their function better, their quality of life gets much better and so that has become my goal as I have gotten more experience.
Locomotor Training (LT)
Locomotor training is a specialized intervention where the patient is suspended in a treadmill over a harness.
Video 3.
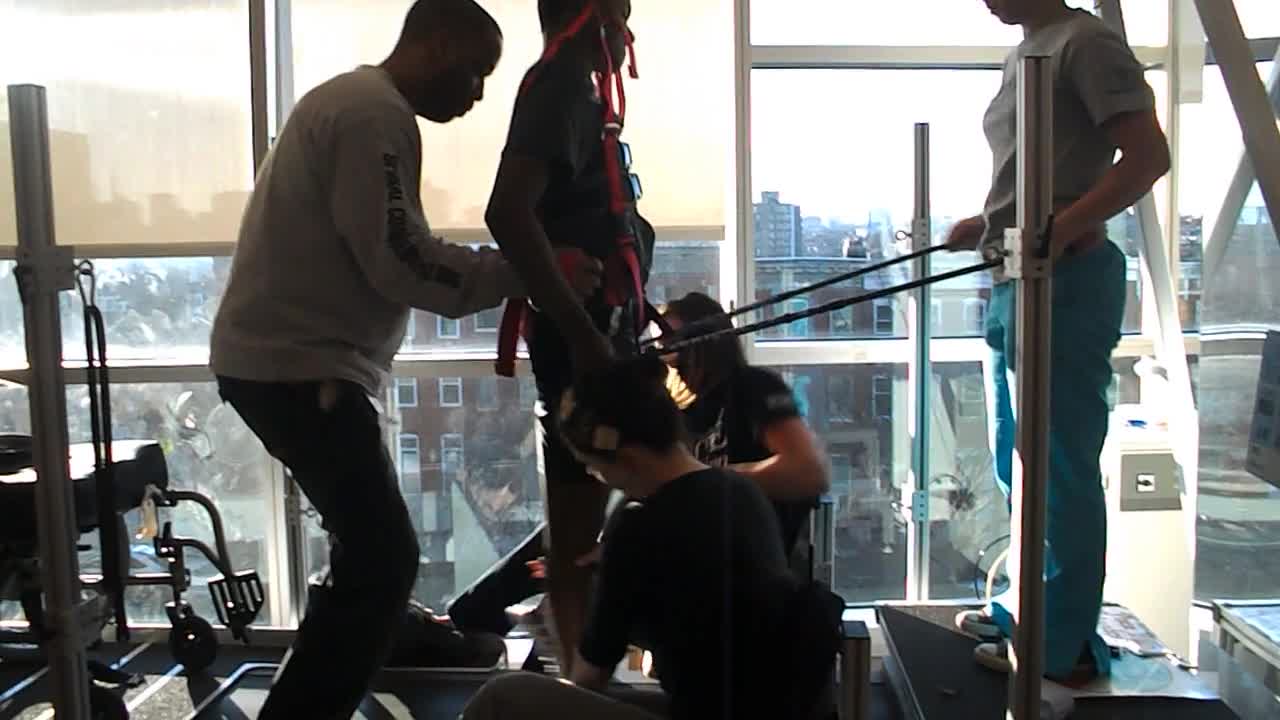
In this scenario, there are therapists that help to move the patient's legs and help support him at the hips. Then, we have another person who is helping with some arm swing. Somewhere in the audience, there is probably an administrator who is calculating the cost of all of this. What you should know is that they are not all therapists. We have techs that we spend a lot of time training to make sure that we can provide this service and facilitation in a way that is feasible for our clinic.
- An activity-based rehabilitative strategy designed to improve sensory, motor and autonomic function, health, and quality of life
- Provides sensory cues to re-train neural patterns that will result in effective locomotion
- Emphasizes recovery of motor function using the intrinsic mechanisms of the nervous system, rather than compensatory strategies
Locomotor training is designed specifically to improve sensorimotor and autonomic functions, health, and quality of life for patients. It focuses on providing specific sensory cues to retrain neural patterns and result in effective locomotion. It also has global health benefits in addition to the benefits of increasing activity in the nervous system.
- Benefits of locomotor training:
- Increased walking speed
- Increased walking independence
- Increased walking endurance
- Improved balance
- Motor recovery
- Decreased asymmetry of gait
- Improve gross motor skills
- Improved well being, life satisfaction, and perceived health
Patients who participate in locomotor training have been shown to have improved walking speed, independence, and endurance. They also have been shown to have improved balance, motor recovery, decreased asymmetry of gait, improved gross motor skills and overall well-being, life satisfaction, and perceived health. Some of the more recent literature about locomotor training has focused on the benefits for the upper extremities and the trunk of that intervention that you just saw. Even though the arms are not that much of a player in the intervention, all of that afferent input and upgoing sensory information is increasing excitation throughout the brain motor cortex and the spinal cord neural circuits to help improve output globally. A friend of mine makes the analogy that when she is training for a marathon and running a lot, her arms start to become more muscular. It is not because she is losing body fat or losing weight, but rather it is because her entire nervous system is getting more activity and that muscle hypertrophy is generalized to other parts of the body.
- Locomotor Training Basics:
- 4 Principles of LT:
- Maximize weight bearing on the legs
- Optimize sensory cues
- Optimize kinematics for each motor task
- Maximize recovery; minimize compensation
- 3 Components to LT:
- Treadmill training
- Overground training
- Community training
- 4 Principles of LT:
There are four principles of locomotor training: maximize weight-bearing on the legs, optimize sensory cues, optimize kinematics for each motor task, and maximize recovery to minimize compensation. There are three components of a session: the treadmill training, overground, and community training. While this sounds really specific, if you squint and use your imagination, you can see that it becomes more generalizable to therapy. Let's reframe this as shown in Figure 5.

Figure 5. Reframing locomotor training to activity-based rehab.
If we think about the principles of maximizing weight-bearing everywhere (not specific to the lower extremities) and optimizing sensory cues and kinematics for each task with the goal to maximize recovery and minimize compensation, it becomes really more of our tenants for activity-based therapy. And, if we think about the components as high-volume repetition, skill practice, and an independent program, it starts to look probably a lot like what you are already doing.
Massed Practice
- Repetitive task-specific and non-task specific activities
- Promote cortical reorganization
- In CIMT, benefits result from the frequency of use of involved side, not the constraint of the uninvolved side
- Repeated multiple times for multiple hours/days
- Improve strength and ROM
- Perfect practice makes perfect
- Incorporate other components
- Principles of LT
- FES
A high-volume repetition might be a situation where we are providing an FES cycling pattern to help prime the nervous system to help with some muscle hypertrophy and help increase spinal excitation. Then, we would spend some time doing skilled practice. This could be a self-feeding task, stacking cans on a shelf, or some home task that we replicate in the clinic. We then ask the patient to go home and practice it. As you start to see what this looks like, it is probably what you are already doing with a little bit of a shift in what your expectations are.
Video 4.

This is an example of massed practice intervention. I published a study a number of years ago where we did sequential stimulation of the wrist extensors and finger flexors, over 30 minutes. You see in the video that when I let the trigger go, the patient's hand closes, and when I push the trigger, the patient's hand opens. The patients would do this for 30 minutes, and then we would do some practical skills training. Tell me how many repetitions you think I did in 30 minutes. The closest answer is 250. The actual repetitions were 210 in 30 minutes. So, patients are getting a lot more repetitions than those 32 we saw a few slides back.
Massed practice is both task-specific and non-task specific. We use components of chunking, rehearsal, and backward training for our functional tasks. It is not reasonable to expect somebody to brush their teeth a hundred times in a session, but you can certainly do a hundred times of reaching across midline or a hundred times of grasp and release in preparation for some of those more functional tasks. I am sure many of you are familiar with the constraint-induced movement therapy which induces a cortical reorganization, The results are from the frequency of use of the involved side, not necessarily because of the constraint of the uninvolved side. It is a use-it-or-lose-it situation. We do this massed practice multiple times a day for multiple hours over the course of weeks. This helps to improve both strength and range of motion by cementing the motor pattern in the brain cortex and helps to strengthen the connections in the spinal cord responsible for that pattern.
- Feasibility in In-patient Rehab
- 15 pts. with UE paralysis s/p CVA in IRF
- 4 days/week of individually tailored UE training
- Ex: lifting cans to a shelf
- Reaching, grasping, manipulating, releasing
- >/=300 reps in 60 min
- 2 days/week of ADL training
Waddell, K. J., Birkenmeier, R. L., Moore, J. L., Hornby, T. G., & Lang, C. E. (2014). Feasibility of high-repetition, task-specific training for individuals with upper-extremity paresis. American Journal of Occupational Therapy, 68, 444– 453. http://dx.doi.org/10.5014/
I promised you some data about this. This was a follow-up study to Catherine Lang's original study out of Washington University. They wanted to see if it was feasible to do high-volume repetition in an inpatient rehab setting. They took 15 patients with upper extremity paralysis post CVA in an inpatient rehab facility and gave them four days a week of individually-tailored upper extremity training. These were things like lifting cans to a shelf. The activities involved reaching, grasping, manipulating, and releasing items, and they aimed for 300 repetitions in 60 minutes. The other two days a week, these patients got regular ADL training.
- Massed Practice Does Not Inhibit Skill Acquisition
- 289 repetitions/session; 47min engaged
- Fatigue was a complaint, pain was not
- Sessions were not often missed
- Improvements in ARAT, grip/pinch strength, UE-FIM
- Pts. with various UE capacities could participate
- Higher doses were associated with better outcomes
- ADL retraining was not sacrificed.
Waddell et al., 2014
What they found was that the massed practice did not inhibit skill acquisition. Patients did 289 repetitions, and while they did not get to 300, they were well above the 32 from before and they did 47 minutes. We all know the reality of inpatient rehab is that 60 minutes might not really happen. What was a surprise was that fatigue was a complaint, but pain was not. Patients did not complain about pain. Sometimes we worry about overuse injuries or that we are going to push our patients too far. However, we have not yet found a limit of how far is too far, and sessions were not often missed. Those of you in an inpatient rehab facility know that half the battle is getting Mrs. Jones out of bed and getting her to want to participate in therapy.
They also found with this high-volume repetition that patients felt more engaged, they did better, and they liked it better. You might be thinking, "What about the FIM scores?" I hear you. Improvements in the upper extremity FIM were equal in the high-volume repetition group and the usual care group. Thus, ADL training was not sacrificed. And when you look at component-level skills via the Action Research Arm Test for grip and pinch strength, patients in the intervention group who got the high-volume repetition did better than patients who were getting usual care.
- Might Take Longer for the UE
- 127 pts. with mod-to-severe UE impairment, >6mos post CVA
- Intensive robotic-assisted therapy (RA)
- Intensive therapist-assisted therapy (TA)
- Usual care (UC)
- Outcomes: Fugl-Meyer Assessment, Wolf Motor Function Test, Stroke Impact Scale
- At 12 wks., RA was better than UC but worse than TA. No significant differences
- At 36 wks., RA and TA were significantly better than the UC
- 127 pts. with mod-to-severe UE impairment, >6mos post CVA
Lo et al.(2010). Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med.,13;362(19):1772-83. doi: 10.1056/NEJMoa0911341.
Now the one caveat here is this study from Washington University in Seattle. They looked at how long those changes take in the upper extremity. They had 127 patients with moderate to severe upper extremity impairments who were chronic stroke patients. They divided them into three groups: a robotic-assisted, a therapist-assisted, and usual care. They tested them with your standard stroke outcomes like the Fugi-Meyer and the Wolf Motor Function Test, these are component-level skills, and then also the Stroke Impact Scale. After 12 weeks of outpatient care, the robot-assisted group was better than usual care but worse than therapist-assisted. So yay, therapist-assisted was best. But at 36 weeks, the robot-assisted and the therapist-assisted were significantly better than the usual care. This speaks to the phenomenon that I mentioned earlier that when patients are given a ton of equipment and told to go home and practice with this at this level, over time they abandon that equipment. They abandon those compensatory strategies and skills because it is just easier to have somebody else do it, but if they can achieve independence at a lower-level skill at something, then they use that something more over time, and movement improves. That is something to think about when you are setting up intervention plans and goals.
Task-specific Practice
Our final component is task-specific practice.
Video 5.

This is a man who had an incomplete spinal cord injury, and he came to me because he had back pain. He was walking with an extensor-dominated pattern and was having back pain as a result. I asked him to stand up, and he did not stand up pretty like this. Instead, he slithered off the edge. It turns out that he did not have any flexion in his system. He had been walking using his extensor tone and head extension to advance his hips. He had no flexion in his system, and therefore, was also having trouble with his biceps and his hand function because he did not know how to bend anything. We took him to the beginning of motor development and practiced things like rolling and sit to stand. Now, he can lean forward and come to stand in a way that is much more efficient and less likely to lead to pain down the road. Task-specific practic is OT's jam. This is where we shine.
- Don't Let Bad Habits Persist
- Use it or lose it: Abhorrent patterns and compensatory strategies have to be overcome by rehabilitation
- Patients will figure out how to get things done (ex: tenodesis)
- Cortical reorganization responds to non-use as much as therapy
- The body learns what we teach it
There is a use-it-or-lose-it phenomenon. Aberrant patterns and compensatory strategies have to be overcome at rehab. The body is going to learn what we teach it. Patients, particularly kids, are going to figure out ways to cheat the system really efficiently. I have never taught a patient how to use tenodesis, but they all do. Cortical reorganization responds to non-use as much as therapy. This concept of learned non-use is that the more we let somebody not do something, the less likely they are to actually do it.
- Train the Affected Limb
- “[In rats,] behavioral experience with the less-affected forelimb early after unilateral [brain] lesions has the potential to increase disuse and dysfunction of the impaired forelimb, consistent with a training-induced exacerbation of learned non-use. These findings are suggestive of competitive processes in experience-dependent neural restructuring after brain damage.”
In rats, behavioral experience with the less-affected forelimb early after unilateral brain lesion has the potential to increase disuse and dysfunction of the impaired forelimb consistent with the training-induced exacerbation of learned non-use. These findings are suggestive of competitive processes in an experience-dependent neural restructuring after brain damage. That is a lot of words to say that if you train the good side, the bad side gets worse. If you focus your effort on the things the patient already has, the things they do not have are going to get worse. In traditional models of rehab when we focus on the spared functions or only on what is available above the level of the lesion, we are actually making their disability worse. The first time I read this, I was like, "Oh no!" But, there is some good news coming. I told you previously that I am an OT that studies walking. Even with all of these specialty interventions and intensity, the recovery of walking still remains elusive to a lot of patients.
Recovery of Walking
The Problem
The truth is that 60% of new spinal injuries are incomplete. These patients have the capacity to walk, and they consistently rate it as a high rehab goal. Intensive gait training is strengthening. Like I showed you with like locomotor training, the results show improved gait speed, endurance, and independence. However, it is expensive based on time and energy as you saw, and it lacks durable results. So, there is still this problem. We still have a problem helping people to fully recover walking in a way that is durable and useful. We know that there are spared connections in the system as I showed earlier. And, we know that those can carry information, but they are not carrying enough or efficiently.

Figure 6. How do we turn up the volume?
The question becomes: How do you turn up the volume in the system? How do you make the spared connections work better? This is where neuromodulation comes in.
Neuromodulation
- Increase excitation in CNS to unmask latent voluntary function
- Introduce some noise into the system to increase the volume on the spared connections
Neuromodulation is an intervention intended to increase excitation in the central nervous system to unmask latent voluntary function. So, we have some baby action potentials that are firing along, but they are not meeting the threshold necessary to show voluntary motion. It is just not enough. We do something. We charge up the system and we introduce some stimulation. We give them some therapy, and now those same small action potentials are able to meet the threshold because we have increased the baseline excitability in the spinal cord. To be clear, we did not change the threshold, but rather, we changed the baseline as a way to show more of the voluntary function. I drew this picture (Figure 7) to explain to a dad of a patient one day about stimulation.
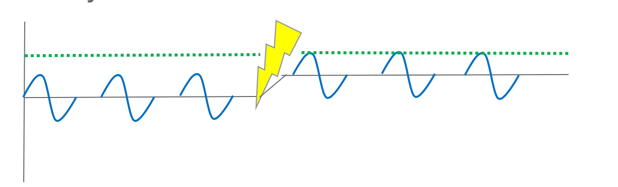
Figure 7. Introducing excitation into the nervous system.
He is an engineer, and he said to me, "Oh you're just introducing some noise into the system to increase the volume on the spared connections." He then said, "It's an electrical problem." I replied, "I guess it is." So in the same way that engineers use electricity to manipulate volume, we are doing the exact same thing in the spinal cord. We are introducing some electricity, some excitation, to manipulate the volume on the spared connections.
Epidural Stimulation
The current field of study is epidural stimulation.
- Microarray implants on the spinal cord
- Different stimulation paradigms to bias different actions
- Research protocols vary
- Training
- Stimulation consistency
- Outcomes are variable
- Surgical risk
Epidural stimulation is microarray implants directly placed onto the spinal cord. They provide stimulation directly to the spinal cord to increase the excitation of spinal neural circuits. Different stimulation paradigms may bias different actions. There is some thought that low-frequency stimulation leans toward extension and high-frequency stimulation causes flexion. Do we need to consider these paradigms at different phases during the gait cycle? This and many other really specific questions are all currently under-researched. There are very small numbers of patients being studied and the training, the stimulation consistency, and the dosage varies. Therefore, the outcomes vary as well. As this procedure requires surgery. It carries a risk.
There are two main centers that are doing these epidural implants. The first is Susie Harkema's lab at the University of Louisville in Kentucky.
- Harkema Protocol
- 4 chronic motor complete individuals
- 16-electrode array implanted at L1
- LT 8-9wks before implantation
- LT 15-85wks after implantation
- Two recovered overground walking
- Only 1 improved ISNCSCI AIS
Angeli et al., 2018
They implanted four individuals with chronic motor complete injuries with a 16-electrode array, which is implanted at level L1. They give patients a lot of therapy. They have eight to nine weeks of locomotor training before implantation and 15 to 85 weeks after implantation. In all, they get well over a year of training around their implant. Of course, you might be scratching your head right now and thinking, "If I give somebody almost a year and a half of therapy, I would expect them to get better." However, we are talking about motor complete individuals who were not expected to recover. The results are that two of her subjects have recovered overground walking. Interestingly, only one has improved their ISNCSCI (The International Standards for Neurological Classification of Spinal Cord Injury) exam or their AIS (ASIA {American Spinal Injury Association} Injury Score). I think this is significant because the interventions we are talking about are not really changing strength, which is what is measured in the ISNCSCI. In contrast, it is changing neural activation.
- Darrow Protocol: E-Stand Trial
- 14 chronic, motor complete individuals
- 16-electrode array implanted at T12
- Sent home with multiple stimulation programs to trial
- Immediate return of voluntary function
- Autonomic function improvement: BP regulation, bowel and bladder, sexual function
Darrow et al., 2019
The other group, the E-Stand Trials, was run by David Darrow out of the University of Minnesota. They implanted 14 chronic motor complete individuals with a 16-electrode array at T12. In these cases, they did not provide any training but sent patients home with an external controller so they could change between multiple stimulation programs to trial different things. So, they might find that one setting helps them to move their legs while another helped them with blood pressure regulation. Or, one program could help with bowel and bladder function. They reported some interesting autonomic function improvements around blood pressure, bowel and bladder, and sexual function, which are all highly-rated rehab goals.
However, both of these protocols are not perfect, and there are surgical risks. I want to now talk about transcutaneous spinal cord stimulation and what we have started to study.
The Solution: TSCS
- Non-invasive, reversible
- Increase in spinal excitability
- Activates spinal locomotor circuits through excitation of dorsal root afferent
- Unmask latent voluntary function
Transcutaneous spinal cord stimulation (TSCS) is non-invasive and reversible. It causes an increase in spinal excitability by activating dorsal root afferents at the level of spinal locomotor circuits to unmask latent voluntary function. I do not want the credit for inventing this. Certainly, people way smarter than I have come up with this and shown that they can, in fact, induce some changes immediately upon application of the stimulation and that we see changes. If we look at this graph (Figure 9) for a second, we can see that time is across the bottom, and the quantity of training and performance is along the vertical axis. We hope that therapy makes us look like this: the more therapy you get, the better you get.
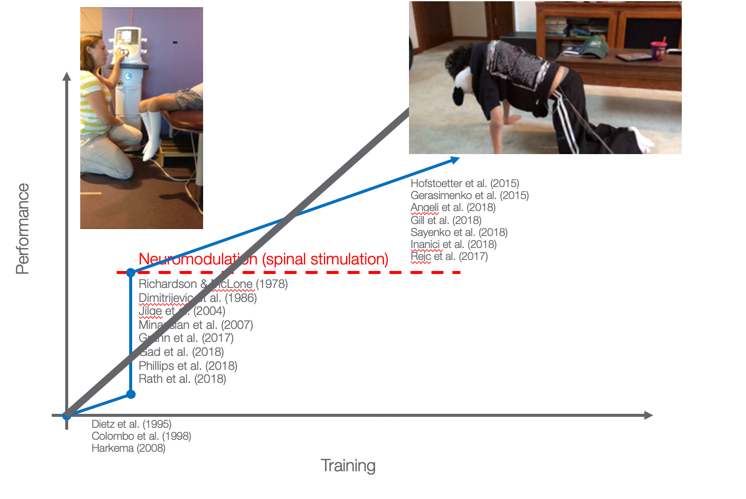
Figure 8. Performance vs. training.
We can make things better, but not a whole lot better with standard therapy. We also have some evidence now to show that with spinal stimulation and neuromodulation, we can make things immediately better.
Video 6.
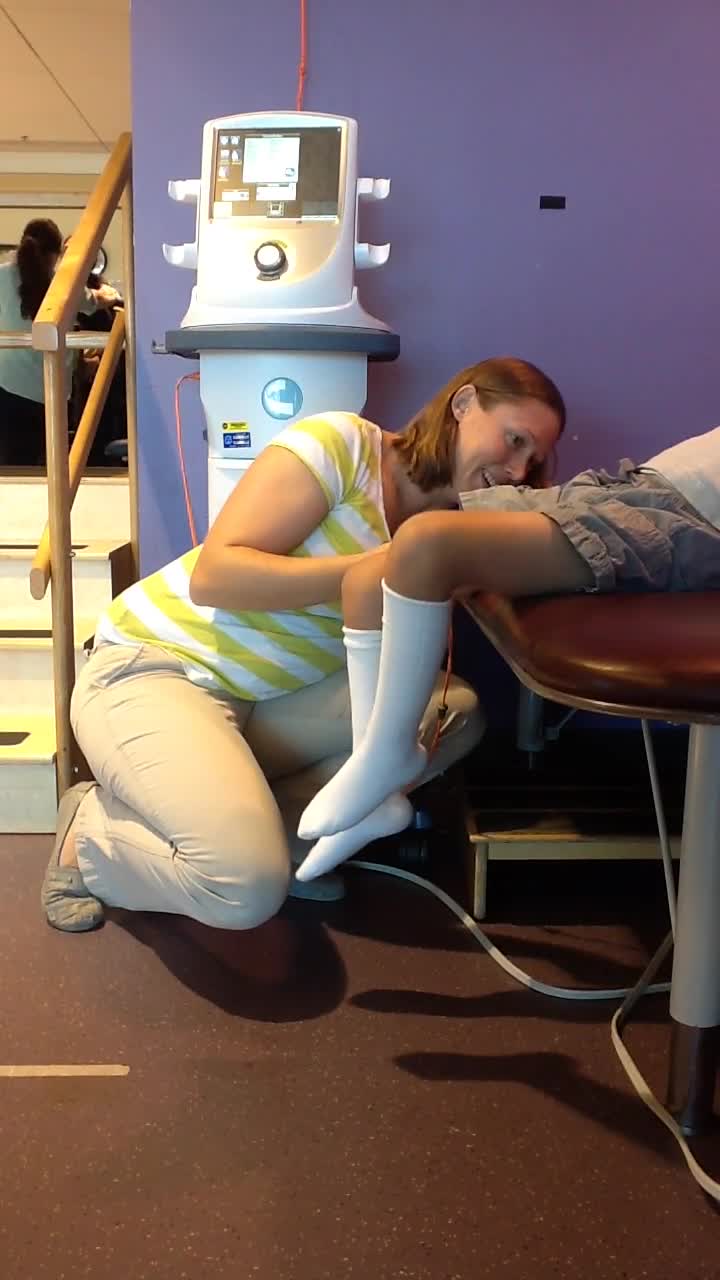
This is a patient who has a motor complete injury. He is getting transcutaneous spinal cord stimulation for the first time. He has one electrode over his spine and two on his abdomen. We are directing the spine through his trunk, and Brook is just turning it up here and taking some measurements. As she goes up higher, watch what happens. Remember this is a kid who cannot move his legs at all on his own. Now all of a sudden, we have hit the circuit that makes it look like he is walking. When Brook stops it, his legs go totally quiet. All of that motion is induced by the stimulation alone. He says, "Oh my gosh, I was running." He was so excited to see his legs move for the first time in 10 years.
If we do neuromodulation repeatedly over time, can we see a change in function? Here is the same kid, in Video 7, who we sent home with the stimulator so that they could continue doing this at home, and he is now able to crawl.
Video 7.

In this video, he had just finished his stimulation. You can see his lead wires are still on, but the stimulation itself is turned off. His dad sent us this video of him crawling across the floor which is something he had not been able to do in 10 years. There is a central pattern generator which is sort of like a mini-brain in the spinal cord, responsible for reflexive reciprocal motion. That is what we are turning on is this central pattern generator. Let's look at this graph again. What happens when you take the stimulation away?
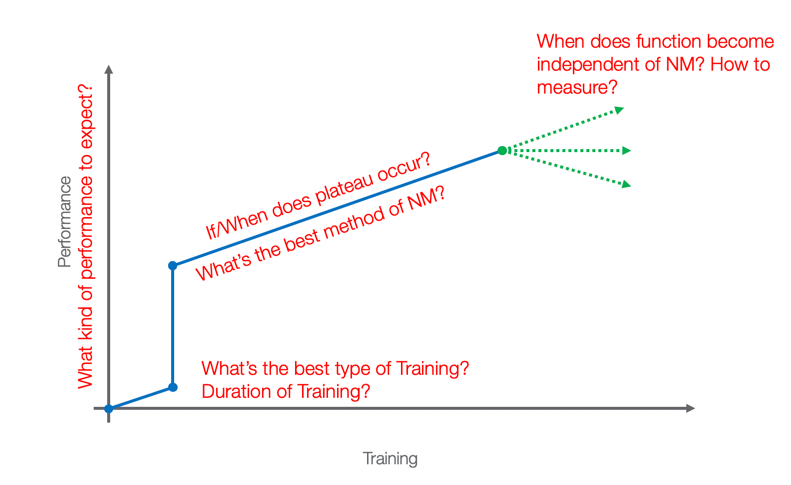
Figure 9. If/when does plateau occur?
If we take it away, do people continue on this trajectory where they were? Or, do they just maintain the gains that they have gotten with the stimulation or start to lose? Like any good researcher, I have lots of questions, but we needed to start with what happens if we combined the transcutaneous stimulation with therapy?
Outcomes
In our study, we had two aims as shown in Figure 10.
Objective.

Figure 10. Aim 2- what is the impact of this on gait training.
The first was to determine the neurophysiologic impact of TSCS within a single session through EMG and dynamometry of the quadriceps at a maximal volitional contraction under both stimulation and sham conditions. Is the stimulation actually doing anything? Aim two is to determine the impact of TSCS and gait training on walking function, and that is what I'll be talking about here.
Method.
- 8 weeks of training: 30 min of TSCS inside a 2-hour therapy session, 3x/week
- 10 MWT, TUG, 6MinWT, WISCI II
- ISCIBDS Bowel and Bladder, Pain
- SCI-SCS
Patients got eight weeks of training with 30 minutes of TSCS inside a two-hour therapy session, three times a week.
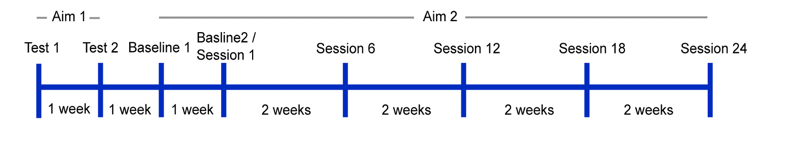
Figure 11. Training overview.
We used standard walking outcome measures like the 10 Meter Walk Test, the TUG, the Six-Minute Walk Test, and the WISCI (Walking Index for Spinal Cord Injury), as well as the International Spinal Cord Injury Basic Data Set for bowel and bladder and pain and the Spinal Cord Injury Secondary Condition Scale (SCI-SCS).
Stimulation Paradigm
- Symmetrical, biphasic waveform
- Commercially available, clinically relevant stimulator
Anybody who is familiar with the literature might at this point be thinking that you have seen some standing studies but you have not really seen anybody walking. All of the research up to this point has been done with a proprietary waveform, a Russian current. Because I am a therapist first, it is super important to me that we do things that are relevant and available to patients now. Thus, we used a symmetrical biphasic waveform in a commercially available clinically-relevant stimulator. It looks like this in Figure 12.
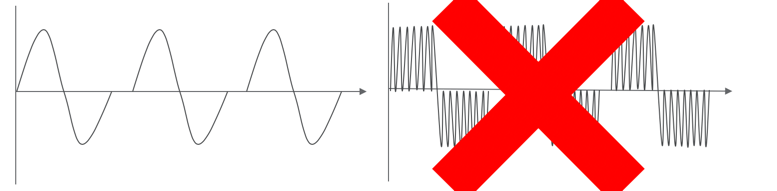
Figure 12. Comparing waveforms.
If you are using any FES, it probably looks exactly like this on the left versus the proprietary stimulation, which is a Russian current. A Russan current has a carrier frequency of 10,000 kilohertz inside the biphasic waveform. Well, that is great, but there are only approximately four simulators in the country that are capable of providing that, and that is not good enough for me.
Video 8.
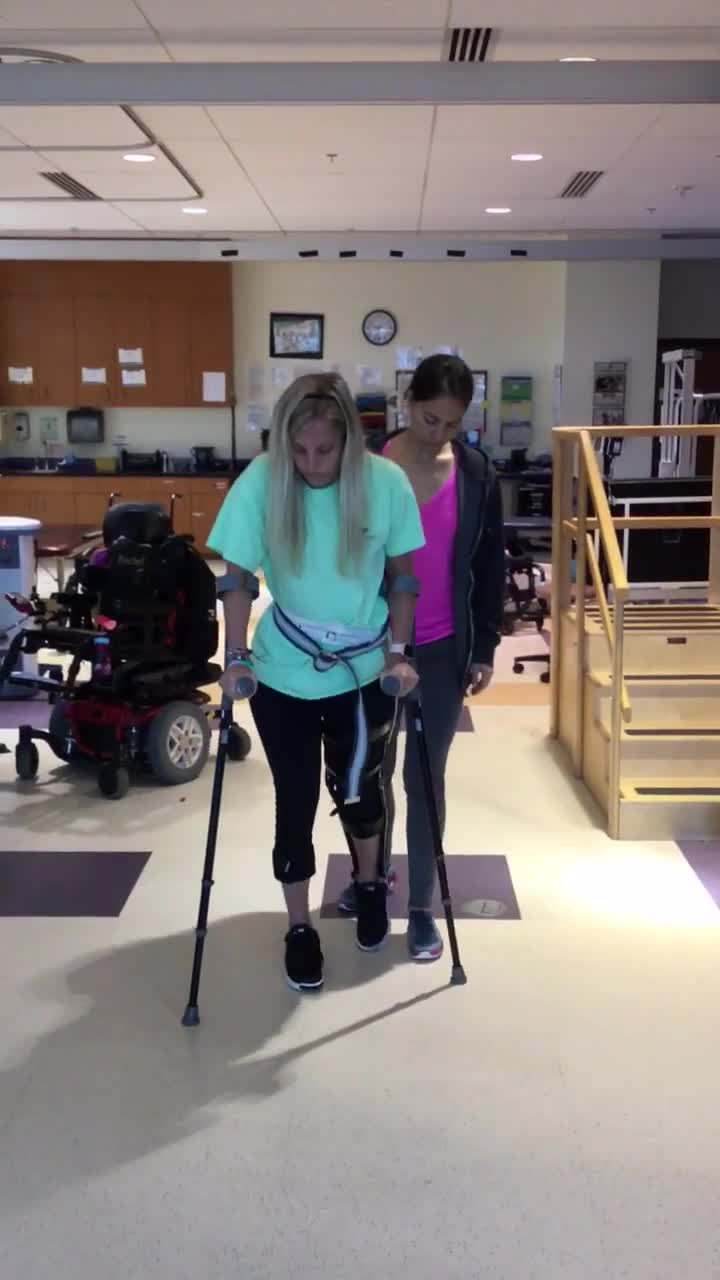
Here is Kristin, and she is walking without the stimulation. You can see that her steps are pretty short and that she has no control in that right knee. It just sort of flaps around or lands in hyperextension. She has to un-weight through her upper extremities, and she is moving pretty slowly.
Video 9.

Here she is now on the same day and in the same session, 20 minutes later. As you can see, she has way more control over her right leg, her steps are longer and faster, and she is not having to un-weight as much through her upper extremities. Look at that control on her right knee as compared to before. It is pretty significant, and for somebody like Kristin, she was able over the course of our study to double her voluntary walking speed without the stimulation. And, she was able to feel secure enough in her walking to transition into a household ambulator.
Demographics and clinical characteristics.
We had 10 subjects. There was a pretty even mix between males and females and a pretty wide range distribution of age and types of injury. What I want to point out is that these patients were all super chronic, anywhere from two to 57 years post-injury. We had an even split between traumatic and nontraumatic, an even split between cervical and thoracic injuries, and an even split between ASIA Cs and Ds. Often, people will say, "Well you made walkers better walkers." That is true, but this is where we have to start.
Findings.
The next few slides show outcomes. (These were not included in the text version of this course due to the proprietary nature of the study--but can be found in the video format.)
Conclusions.
- Safe and feasible in an outpatient setting
- Improvements in all outcome measures
- Immediate, lasting impact on spasticity
- Latent improvements in voluntary function
If you remember back to the epidural studies of a year plus of stimulation, in contrast, we are making changes just over 18 visits. The transcutaneous stimulation might not be perfect for everybody as there were some patients that remained flat with no improvement, and we will start to sort out why that is. However, overall, there were improvements in all of the outcome measures.
So, we start to ask the questions about:
- What makes somebody stay flat?
- Who becomes a responder and a non-responder?
- How can we decide for whom this intervention is most appropriate?
Spoiler alert, I do not have the answer to any of those questions because we only had 10 subjects. It was not enough really to do any sub-analysis, but it starts to guide our research for the future. We did have a number of additional findings. Half of the subjects reported a recovery in voluntary voiding of bowel and bladder. About two-thirds reported improvement in pain, both musculoskeletal and neuropathic, but very few subjects reported improvement in sensory function for light touch, pinprick, and vibration. This was a surprise to us considering the improvements in pain. The stimulation was safe and feasible in an outpatient setting. We used a commercially-available device to deliver safe and feasible stimulation for patients who need it now. They had statistically significant improvements in all outcome measures, as well as an immediate and lasting impact on spasticity and latent improvements in voluntary function.
Why OTs Should Care About Walking
- Return to kinematically normal activity
- Greater accessibility
- Greater generalizability of skill
- Increased activation of UE motor cortex in a weight-bearing position
Why should OTs care about walking? We want to help return patients to kinematically normal activity. I do not know about you, but I walk across the room to go to the bathroom, I stand to brush my teeth, et cetera. Also, it provides greater accessibility for our patients and greater generalizability of skill. Again, if we can help somebody recover walking, it improves their ability to do their ADLs and not need accessible settings to do so. There is also a lot of literature to show increased activation of the upper extremity motor cortex in the lower extremity weight-bearing position.
Impact of TSCS on UE function
- Chronic, cervical motor complete
- Post-6-months of inpatient rehabilitation remained dependent
- TSCS + intensive gross/fine motor training
- Recovered voluntary function and sensation
Inanici et al., 2018
That afferent input will also help your upper extremity outcomes. There is a group working on TSCS and upper extremity function. They looked at chronic cervical motor complete patients who were post six months of inpatient rehab but remained dependent. In this particular intervention, they received TSCS plus intensive gross and fine motor training. They found that this patient also recovered voluntary function and sensation. This is your mandate, and this is what you need to go out and do.
"Pump Up the Jam"
- Incorporate high volume repetition during stimulation.
- Do as much as possible in an upright and weight-bearing position.
- Then do more.
It is time to pump up the jam. We need to give our patients more and change what it is we are expecting from them, whether it is with therapy, whether it is with spinal cord stimulation, whether it is with vibration or any kind of activity that is going to increase the excitation of the spinal cord so we can see better outputs. The things you can do right now is to incorporate high-volume repetition during stimulation. Try the addition of FES. Try the addition of even just sensory-level stimulation as a way to increase cortical awareness, and then do high-volume repetition during stimulation. What I ask my therapists all the time is, "Can you do one more?" Every session you approach, you should think, "How can I do it closer to normal?" Then secondly, do as much as possible in an upright and weight-bearing position. And then, do more. My patients get out of their wheelchairs as much as possible, We use vibration, tilt tables, and supported standers
Thanks for your time today.
Acknowledgments
- The patients and their families
- Liza McHugh, PT and Ashley Miller, PT
- Kristan Leech, PhD, PT
- Amy Basitan, PhD, PT
- Cristina Sadowsky, MD
None of the research is done alone. I want to thank the patients and their families because, without them, none of this is possible. I also want to thank my research team who is also super valuable to me.
References
Lang, C. E., Macdonald, J. R., Reisman, D. S., Boyd, L., Jacobson Kimberley, T., Schindler-Ivens, S. M., . . . Scheets, P. L. (2009). Observation of amounts of movement practice provided during stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 90, 1692–1698. http://dx.doi.org/10.1016/j.
Lo et al. (2010). Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med.,13;362(19):1772-83. doi: 10.1056/NEJMoa0911341.
Waddell, K. J., Birkenmeier, R. L., Moore, J. L., Hornby, T. G., & Lang, C. E. (2014). Feasibility of high-repetition, task specific training for individuals with upper-extremity paresis. American Journal of Occupational Therapy, 68, 444– 453. http://dx.doi.org/10.5014/
Additional References
In the attached handout.
Citation
Martin, R. (2020). Functional electrical stimulation for OTs: Principles and application. OccupationalTherapy.com, Article 5203. Retrieved from http://OccupationalTherapy.com
