Editor's note: This text-based course is a transcript of the webinar, Changing The Brain: Using The Principles Of Neuroplasticity And Motor Learning To Improve Functional Outcomes In Acquired Brain Injury, presented by Angela Reimer, OTD, MOT, OTR, CBIST.
*Please also use the handout with this text course to supplement the material.
Learning Outcomes
- After this course, participants will be able to examine basic motor learning theory and its link to neuroplasticity.
- After this course, participants will be able to apply motor learning theory to develop appropriate occupation-based interventions.
- After this course, participants will be able to analyze the effect of meaningful occupation on neuroplasticity.
Introduction
Thanks, everybody, for taking time out of their day today to talk with us about motor learning and neuroplasticity.
Prevalence
When we begin talking about brain injury and stroke, it’s important to acknowledge just how common these events are—far more common than many people realize. I often don’t grasp the full impact until I sit down and look at the numbers.
In the United States alone, 2.8 million people experience a traumatic brain injury (TBI) each year. And strokes occur at an astonishing rate—every 40 seconds, someone in the U.S. has a stroke. Just imagine setting a timer during this course, and hearing it go off every 40 seconds—that would represent another person experiencing a stroke, just within our borders.
In addition to TBIs, 795,000 people sustain acquired brain injuries from non-traumatic causes each year. But I’ll be honest: that number is likely an underrepresentation. When we look at mild traumatic brain injuries, especially concussions, we lack consistent reporting mechanisms, and many cases go undiagnosed or misdiagnosed. You know this already—especially if you’re working in neurorehabilitation. A significant portion of our caseloads, particularly in neuro-focused practice settings, involves individuals who have experienced some form of acquired brain injury, whether from trauma, stroke, or another cause.
As we move forward, we're going to start diving into neuroplasticity. It's a term we hear often—it’s become a buzzword. But the real question is: what does neuroplasticity mean? And more importantly, what does it mean for us as clinicians, educators, and therapists working in this space? Let’s start to unpack that.
Neuroplasticity
What Is It?
When we talk about neuroplasticity, we're referring to the brain's remarkable adaptive capacity—its ability to change, reorganize, and form new connections within the central nervous system. Neuroplasticity is not just a concept; it’s a deeply researched and evidence-based truth that the brain can change, and that meaningful, functional improvements are possible even decades after a stroke or brain injury. We, as therapists, know this. A wealth of research supports the idea that neuroplastic change can occur 20 to 30 years post-injury.
Yet despite all of this, many patients are still being told by medical professionals—sometimes family physicians, neurologists, or others within the healthcare system—that the brain’s window for change is only six to twelve months. I still have patients walking into my clinic in panic mode, believing they’re racing against a ticking clock. They’ve been told they only have a year to progress, and they arrive overwhelmed, thinking, “We have so much work to do in such a short time.”
As therapists, part of our responsibility is education—specifically, educating our patients and their families about the realities and misconceptions surrounding neuroplasticity. Neuroplasticity slows down over time, but it does not stop. It is lifelong. That message alone can shift a person’s entire outlook on recovery.
We also need to ensure patients understand their role in the process. Neuroplasticity doesn’t happen passively. It is driven by experience, effort, repetition, and engagement. Our clients need to know what they can do to influence change and that they are active agents in their rehabilitation—not just recipients of care.
And here’s another critical point: as a medical system, we tend to front-load patient education. For instance, we flood patients and families with information right after a stroke. But that acute phase is also chaos and shock—patients are overwhelmed, and families are still processing what’s happened. Very little of that education sticks.
This is why it’s so important that we, as therapists, revisit and reinforce education throughout the continuum of care. Whether someone is in inpatient rehab, outpatient therapy, or even years into their recovery, we need to keep offering information in a way that meets them where they are. That way, they can become informed, confident, and empowered partners in their rehabilitation journey. And that, ultimately, is what helps drive long-term success.
Animal Research
When we talk about neuroplasticity, it's helpful to return briefly to some of the foundational animal research that shaped the principles we use in therapy today. Although this research is older, it's incredibly relevant. I often share these stories with patients, not by handing them the studies but by explaining them in a way that helps them understand the science behind their recovery.
One of the key researchers in this area was Dr. Edward Nudo, a groundbreaking neuroscientist. He observed that monkeys naturally use both hands in their daily activities—their little “monkey ADLs”—whether eating or grooming each other. They didn’t favor one hand over the other. This led Nudo to ask, “What would happen if one of the monkey's hands became more difficult to use?”
In his experiments, he deafferented one of the monkeys' hands, meaning he made it harder for them to use that hand by disrupting its sensory input. He found that when the monkeys had difficulty using the impaired hand, they stopped using it. Instead, they began to favor and rely more on the unaffected hand. This behavioral shift is what we now call learned non-use.
Learned non-use is now recognized as one of the most significant barriers to functional recovery after brain injury or stroke, particularly in the upper extremity. It's not just spasticity or weakness that limits movement—it’s the brain adapting by not using the limb, reinforcing its disuse. The brain stops assigning as much cortical space to the impaired limb because it’s unused.
Nudo didn’t stop there. In the next phase of his study, he restrained the monkey’s unaffected (or “good”) limb and observed what would happen. Remarkably, once the dominant limb was restricted, the monkeys began to use the previously deafferented, impaired hand again. And when the restraint was removed, they resumed using both hands.
He followed this behavior with brain imaging and found something even more powerful: once the monkeys began using the impaired limb again, the cortical representation of that limb increased. The brain was reorganizing itself in response to use—a fundamental demonstration of neuroplasticity.
This research laid the foundation for what we now call constraint-induced movement or modified constraint-induced therapy (CIMT or mCIMT), which is widely used in stroke rehabilitation and upper limb recovery.
But Nudo's work didn’t stop with just restraint. He and others continued to study what variables influence plasticity. They compared groups of monkeys using different methods to obtain food. One group had easy access to food in a shallow dish and used a broad, raking grasp. The other group had to reach into a deep container, requiring a more refined pincer grasp to retrieve the food. Brain scans later revealed that the group that had to use more skilled, precise movements—more challenging motor tasks—showed greater cortical reorganization and more robust neuroplasticity.
These studies underscore two major points: first, use matters—we must encourage patients to use their impaired limbs in meaningful ways. Second, challenge matters—the complexity and precision required in a task drive even greater neuroplastic change.
This is the basis for so much of what we do in upper limb rehabilitation. We're not just promoting movement but carefully designing experiences that engage the brain, foster reorganization, and support proper recovery through meaningful, skill-based, and appropriately challenging tasks.
Principles of Neuroplasticity
In our work with neuroplasticity and motor learning, we rely on several foundational principles that guide how we engage the brain in therapeutic change. One of the most important is the concept of “use it or lose it.” This principle ties directly into learned non-use. If a patient stops using their impaired limb, particularly the upper extremity, the neural pathways supporting that function begin to weaken. Conversely, “use it and improve it” means that with consistent practice and training, we can strengthen those pathways and improve function.
Specificity is another key principle. Task-specific training matters. It’s not enough to move an object—what the object is and how it relates to the patient’s real-life situation are essential. For example, grasping a small teacup requires different hand shaping than grasping a large coffee mug. Using the patient’s own household items whenever possible ensures that the motor patterns being trained are meaningful and directly applicable to their daily lives.
Repetition is vital. While we don’t know the exact number of repetitions needed to create lasting change in the brain, we know that it takes tens of thousands of reps to drive neuroplasticity. Most patients are not getting enough repetition within clinic sessions alone. That’s why home exercise programs—even very early on—are crucial. Patients need frequent, meaningful practice to create the neural change we aim for.
Intensity matters, too. Research supports the idea that high intensity—meaning both high repetition and high challenge—is more effective at driving neuroplasticity. We’ll explore this more later, but the key takeaway is that pushing the system, within reason, enhances outcomes.
Timing is also an essential factor. The brain shows its most significant plastic potential in the first one to three months post-injury. During this window, we often see rapid improvements. From three to twelve months, gains continue, but the rate of change slows. After twelve months, plasticity remains possible, but the pace of change declines even further.
Age influences plasticity as well. Younger patients typically have more capacity for neuroplastic change than older individuals, due to age-related changes in cortical structure and function. Meaningful recovery is still possible at any age, with different expectations and pacing.
As occupational therapy practitioners, salience is one of the most important principles we champion. If an activity isn’t meaningful to the patient, it likely won’t generate strong neural change. The goal is to tie therapeutic tasks to what matters most to the individual. This is especially critical when working across age groups or with diverse populations—goals and motivations differ, and therapy must reflect that.
We also consider transference—the ability to transfer skills learned in one context to another. Training one task can activate neural pathways that support performance in untrained tasks. This can be enhanced through strategic planning of therapeutic activities and adjunctive interventions.
Emerging research is now exploring ways to boost transference and neuroplastic potential through non-invasive brain stimulation techniques, including neuromuscular electrical stimulation (NMES), transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS). These tools may enhance the effects of therapy when used appropriately alongside functional task practice.
Finally, we must be mindful of interference—the idea that neuroplasticity can be blocked or disrupted. Learned non-use is a prime example. If a patient continues to rely exclusively on their unaffected limb, and we don’t actively engage the affected side, the brain won’t rewire to support improved use of that limb.
These principles—use it or lose it, specificity, repetition, intensity, timing, age, salience, transference, and interference—are the foundation for understanding how and why neuroplastic change happens. Keeping them in mind allows us to design more effective, individualized, and evidence-based interventions that truly support functional recovery.
Does Function Matter?
When we start talking about function in the context of neuroplasticity and motor learning, the answer to whether it matters is a resounding yes, especially for us as occupational therapists. Function isn’t just the foundation of our practice; it’s also a powerful driver of neuroplastic change. And the research supports this in meaningful and practical ways.
There have been several studies over the past few decades—some of them foundational—that continue to influence how we think about functional outcomes in therapy. As therapists, we often select interventions based on two key influences: our clinical expertise (which includes what we learned in school or through continuing education) and our patients’ observable impairments. For example, if a patient presents with spasticity, our default may be pulling out the go-to tools or techniques we’ve used for spasticity. That’s understandable, but it’s not always guided by current research or by what’s most meaningful to the patient.
Interestingly, these studies found that stroke survivors' treatment preferences—the actual goals and values of our clients—often ranked lowest in influencing intervention selection. And yet we know that what’s meaningful to the client is exactly what drives salience, which is one of the key principles of neuroplasticity. It’s not about what I think my patient should work on; it’s about what they find valuable, motivating, and functional.
One study by Trombly and Lin Wu illustrated this beautifully. Participants were divided into two groups: one rang a bell before performing a line bisection task, and the other touched a lever. The group that rang the bell had significantly better movement kinematics. Why? Ringing a bell has a functional outcome. It offers auditory feedback. The action has purpose and consequence, and that purpose enhances their motor control.
Another study by Vollmer had three groups reach for different objects: a piece of tape on the wall, a light switch that didn’t work, and a working light switch. The group that turned on the functioning light showed the most improvement in motor performance. Adding a sensory cue like a light switch without function didn’t significantly change performance, but motor performance improved once there was a real, purposeful outcome (turning on the light).
In a related study, participants were asked to identify two tasks they liked the most and two they liked the least from a list. Then, they were asked to perform these tasks under purposeful and non-purposeful conditions. Researchers monitored repetitions, heart rate, blood pressure, and perceived exertion. Surprisingly, it wasn’t how much they liked the activity that made the biggest difference—it was whether or not the task felt purposeful. During non-purposeful versions of even the most preferred tasks, heart rate and perceived exertion increased. Tasks felt harder and more taxing when they lacked functional meaning.
Finally, Woo and Trombly explored what happens when a person uses a food chopper. In one group, people used the chopper on a blank piece of cardboard. In the other group, they chopped an actual mushroom. The group working with real food had better reaching and motor patterns, again demonstrating that function drives better movement quality.
So what do all these studies tell us? Functional tasks matter—not just because they’re practical, but because they directly impact kinematics, motor planning, motivation, and neuroplastic change. These internal and external factors combine to produce outcomes that simulated or non-purposeful tasks can’t match.
As therapists, we must go beyond technique and impairment-driven treatment. We need to anchor our interventions in function and in what the task means to the patient. That’s where neuroplasticity is truly activated, and that’s how we help people move better and live better.
Principles of a Task-Oriented Approach
The task-oriented approach emerged from this growing body of research and clinical observation and has become foundational in occupational therapy practice, especially in neurorehabilitation. The core of this approach rests on the understanding that the practice of a movement results in improvement of that movement, not just any purposeful, meaningful, and challenging practice that drives both motor learning and neuroplasticity.
We know repetition matters—a lot of it. We need large amounts of meaningful practice to master a motor skill. But we also know that repetition alone isn’t enough. Learning occurs when there is integration—when the brain must solve problems in real time, not just perform memorized motions. So yes, repetition is necessary, but challenge is critical.
Let’s say a patient can reach out and press a Big Mac switch to activate a toy for their child. That’s great—but once they’ve mastered that movement, you’re no longer promoting motor learning. The skill has become automatic. At that point, it’s time to up the challenge. Add weight to the limb, use resistance bands, change the surface or environment, have them do it faster, blindfolded, or while managing a cognitive task. Every variation presents a new problem to solve, which drives further neural change.
Feedback is another key ingredient in learning. And it’s not just, “You’re doing great.” While encouragement is helpful, it’s not sufficient for motor learning. Feedback should be precise, functional, and frequent—“Let’s bring that elbow in a bit,” or “Try reaching an inch farther before initiating the grasp.” Beyond verbal, consider visual feedback like video playback. Let patients see themselves move. It supports metacognition and fosters self-efficacy, helping clients become more actively engaged in their recovery.
Motivation and engagement fuel neuroplasticity. If a task feels dull or meaningless, the brain won't invest. But if it feels purposeful and relates directly to the patient’s daily life, it becomes an opportunity to build lasting change.
This is why variable practice is so important. We don’t want patients to perform tasks only in one setting under ideal conditions. We want generalization—for them to succeed in real life, across environments. That’s why, for something like toilet transfers, you might spend an entire 45-minute session touring every bathroom in a hospital, practicing on different toilets with different heights, layouts, and grab bar placements, because your ultimate goal isn’t success in the clinic. It's success at Target. At their daughter-in-law’s house. In their real world.
Massed practice, or performing a skill intensively over a short time, often promotes better motor learning than distributed practice, which spaces out repetitions. Doing 20 reps in one session may be more effective than doing five reps across four sessions, though this depends on patient tolerance and context.
Finally, whole-task practice is more effective than partial-task practice—again, because function matters. Practicing the whole sequence of making a peanut butter and jelly sandwich is more impactful than practicing isolated parts, like just opening the jar or spreading peanut butter. The full task has meaning, relevance, and functional payoff, whereas partial practice may feel disjointed and less purposeful.
This underscores what we’ve seen repeatedly: the brain learns best through functional, meaningful, and challenging activities performed with intensity, variety, and feedback. That’s how we support true recovery—and why occupational therapy is uniquely equipped to lead this process.
Integrating Concepts Into Practice
These concepts are essential to remember as we plan treatment sessions and critically consider our interventions. Understanding neuroplasticity, task-oriented approaches, motor learning principles, and the power of function is only valuable if we can translate it into clinical action. So, the next question becomes: How do we integrate this into practice? What does this look like in a session tomorrow?
Let’s be honest—if we don’t start applying it right away, the likelihood that we will ever will begins to drop. Each day that passes without integrating these approaches into our work represents a missed opportunity for our growth as clinicians and our clients' potential for recovery. That’s why it’s crucial to begin now.
Start by asking:
What is meaningful to this client?
How can I make this task functional?
Am I including enough repetition and challenge?
Am I giving feedback that supports learning, not just encouragement?
These are small but powerful shifts in how we think, plan, and engage with our patients. Whether it’s choosing a purposeful object to reach, turning a rote exercise into a problem-solving task, or varying practice conditions to promote generalization, these adjustments bring theory to life and make our interventions more effective.
So, yes, begin using this tomorrow. Start small if you need to. But start—because the brain is ready, and so are your clients.
Motor Learning Integrates Research
When we consider motor learning, it’s important to understand that the foundational research and theory come from a variety of disciplines—psychology, neurology, physical education, athletics, and rehabilitation. As occupational therapy practitioners, we naturally draw from and integrate these fields into our daily clinical reasoning.
Motor learning theory focuses on how people acquire or relearn skills involving movement and coordination. Whether it’s learning to walk again post-stroke, regaining the ability to reach and grasp, or refining fine motor control, motor learning gives us the framework to structure practice, feedback, and progression to support meaningful, long-term improvement.
The P.R.A.C.T.I.C.E. Principles: Common Ingredients for Efficacious Stroke Rehabilitation
- Part-whole practice
- Repetitive, task-specific, and goal-focused
- Activities should be meaningful to the client
- Client-driven – goals and content of practice
- Train in a practical way
- Emphasize accomplishments and awareness – copious, diverse feedback, self-efficacy, home programs
As we dive into motor learning, we see how closely these practice principles align with the task-oriented approach and the core principles of neuroplasticity. Much of what we've already discussed ties together here, especially when it comes to designing treatment that’s not only effective but also meaningful.
We know the difference between part versus whole practice, and we recognize that repetitive, task-specific, goal-directed training leads to better outcomes. These are foundational truths. But here’s the catch: repetition alone won’t stick unless the activity is meaningful to the client. If a patient doesn’t understand why we’re doing what we’re doing, we lose their attention, their investment, and often, their progress. This becomes even more critical when we’re addressing perceptual or cognitive impairments, like apraxia, neglect, or visual-spatial deficits. It can be tempting in those scenarios to reach for paper-and-pencil tasks or abstract tabletop activities. But unless those tasks are relevant and contextualized, we risk missing the mark.
Our interventions must reflect what truly matters to the person in front of us. Every evaluation and treatment session should start with questions like, "How are things going at home?" or "What’s driving you crazy this week?" These answers direct the session in a way that is not only therapeutic but also personal. If a client says, “I tried to bake a cake this weekend and couldn’t hold the bowl still while I stirred,” that becomes our session. We move to the kitchen. We work on that exact task because that’s where motivation, effort, and neuroplasticity come together.
Our goals should be client-driven, grounded in what clients want, not just what we think they should achieve. During evaluation, this means asking about functional priorities and integrating them into the treatment plan immediately, not as an afterthought but as the core of our therapeutic direction.
Training should be practical, relevant to the home, transferable to the real world, and reflective of our patients' challenges. Feedback should emphasize accomplishments and build self-awareness, promoting self-efficacy. When patients begin to believe in their progress, they are more likely to stay engaged, persist through frustration, and continue working toward independence.
Finally, we need to support this effort beyond the clinic walls. Home programs are not just about assigning exercises; they enable action and participation in the patient’s real life. They ensure that practice continues, that learning is reinforced, and that the client sees themselves as active participants in their recovery.
These aren’t just best practices—they are the bridge between knowledge and transformation.
Where Do I Start?
You might think, "Okay, great info, Angie—but how do I do this in practice?" That’s the key question. If we don’t start applying this now and delay integrating it into our daily treatment sessions, the likelihood that we ever do gets smaller. So let’s talk about how to begin weaving this into your sessions, starting tomorrow.
First, start with history. I spend 20 to 30 minutes gathering a detailed history during my outpatient evaluations. I ask questions like: What did you enjoy doing? What was your job? Did you drive? Did you use a computer? Do you have children? How old are they? These questions aren’t just small talk—they help me build an occupational profile. This profile becomes my roadmap for treatment because it tells me what is meaningful to the client and what will keep them motivated.
Then, ask about the patient’s goals. And I mean their goals, not yours. This is where so many of us can go off track. We look at someone in a wheelchair and immediately think, “We need to work on transfers, dressing, grooming, toileting…” And sure, those are important. But are they essential to that person?
Let me give you an example. I once had a patient, a very well-off gentleman, who came into outpatient rehab dependent on nearly everything. He used a wheelchair with a half-lap tray, and his private caregiver, Bernard, managed his daily needs. From a clinical perspective, I saw ADLs and self-care as top priorities. However, none of it mattered when I asked the patient what he wanted to work on. “I’ve got Bernard,” he said. His goal? He wanted to be able to hold his book open during services at his temple. That was it. I would have missed what mattered if I had imposed my own goals. Instead, we worked on what was meaningful to him, which drove his engagement.
Use activity analysis. Remember those assignments in OT school where we had to analyze every skill needed to carve a pumpkin or make a sandwich? They may have felt tedious then, but now they’re vital. When watching a patient attempt a task, don’t just see failure—analyze. What’s not working? Is it a lack of pronation, poor grasp strength, timing issues, or decreased postural control? Activity analysis lets you precisely break down the components and build your treatment plan.
Evaluate your tools. I’m not saying you can never use cones or pegs—there’s a time and place. But ask yourself why you’re using them. If you aim to work on weight shifting, do you need to reach for a cone? Or could you have your client set the table by moving plates, reaching across the table, and adjusting utensils? That’s still weight shifting, but it’s embedded in a functional, meaningful task that transfers more naturally to real life.
Make it functional, and make it collaborative. Involve your patient in the process. Explain why you’re choosing a certain task. Check in at the start of each session: “How’s it going at home? What’s frustrating you this week?” If your patient says, “I couldn’t hold the bowl while I tried to stir a cake batter this weekend,” then that becomes your focus. You’re not guessing—you’re aligning the session with something meaningful for the client.
This approach doesn’t just enhance outcomes—it creates a therapeutic alliance built on respect, relevance, and shared purpose. And that’s the real power of occupational therapy. When we root our interventions in the patient’s story, their goals, and their daily life, we transform therapy from routine to transformational.
Life History and Occupational Profile
As we’ve already started to explore, a client's life history and occupational profile are essential elements of effective, meaningful, and neurologically-informed care. When I talk about gathering an occupational profile, I’m not just checking a box. I’m asking about the whole person—What are your hobbies? What did you do for a living? What does your family look like? What’s your home environment like? What are your values, your habits, your routines? These are not filler questions. They are the foundation of everything that follows.
This becomes especially critical in cognitive rehabilitation. Habits and routines are encoded and stored differently from non-habitual actions in the brain. They’re often more resistant to damage, making them incredibly valuable recovery resources. When we anchor therapy in routines that are already hardwired—like how a person gets dressed, prepares their morning coffee, or organizes their workspace—we’re tapping into preserved pathways and increasing the likelihood of success. Conversely, if we ignore these patterns and try to teach something new that isn’t contextually grounded, we may see resistance, frustration, or failure.
Even the smallest detail can make a big difference. If someone has always started their day by walking around the block or reading the paper in a particular chair at a specific time, lean into that. The more detailed and personalized your understanding of the client, the easier it becomes to plan relevant, engaging, and functionally effective treatments.
And here's something that often gets missed: ask the hard questions. Don’t be afraid to go there. Ask about mood, mental health, and emotional well-being. Research continues to show that one of the most frequently cited unmet needs among stroke survivors and their caregivers is support around psychosocial determinants—yet these are the very areas that too often go unaddressed. Physicians are often short on time, and other providers may hesitate to explore emotional concerns. That’s where we come in.
Occupational therapy practitioners are well-positioned to create a space for these conversations. We see clients over time, build trust, and approach care holistically. By asking questions about depression, anxiety, motivation, and emotional fatigue, we give our clients permission to acknowledge these struggles and integrate them into the recovery process. That doesn’t just improve therapy—it improves quality of life.
So the takeaway is this: the more information you gather, the better your outcomes will be. Be curious, thorough, and brave. Ask the questions no one else is asking, and use the answers to build a treatment approach centered on the person, not just their diagnosis.
Evaluation Considerations
As we transition into the evaluation phase, the central focus must remain on the client’s experience and perspective. The evaluation isn’t just a checklist of impairments—it’s our opportunity to understand what truly matters to the person in front of us. We want to ask: What does the patient value? What frustrates them? What do they need to be able to do in daily life? These three guiding questions should shape our evaluation, our observations, and ultimately, our intervention planning.
When selecting and administering assessment tools, we focus on identifying how the patient’s current deficits connect to functional limitations and, from there, how we can translate that information into meaningful goals. Every test or screen should serve a clear purpose in helping us understand the person's occupational challenges. But assessment alone isn't enough.
We must actively involve the patient and their caregiver in evaluation and goal setting. These conversations should be collaborative. We’re not just identifying problems—we’re developing shared priorities. This approach supports motivation, fosters buy-in, and ensures that we’re working toward goals that the patient sees as valuable.
Just as critical is the role of education for both the patient and the family. While education often begins in the acute care setting, it’s important to remember that the timing isn’t always ideal. Families and patients are overwhelmed in the hospital. They may hear the information, but they’re not in a place to fully absorb it. That’s why we must continue education throughout the entire continuum of care, particularly in post-acute settings like outpatient rehab.
One of the simplest yet most effective ways I begin this education is by asking, “Do you know where your stroke occurred?” The most common response I get is a vague guess: “I think it was on the left side of my brain.” At that point, I’ll pull up a visual of the brain and walk them through it: “Here’s where your stroke happened. This is the area of your brain that is affected. And here’s why you’re having the symptoms you’re experiencing.” That moment of connecting brain function to real-life symptoms is often powerful for both patients and caregivers. It answers that unspoken question: Why do I look or function differently than someone else who also had a stroke?
These educational moments build insight, reduce fear, and increase patient empowerment. And that’s not just good practice—it’s essential. We also need to educate patients on what they can do at home to take ownership of their recovery. What are the practical things they can work on between sessions? What strategies can they use to support function and minimize frustration?
When we do this well—when evaluation is collaborative, functional, and rooted in education—we set the tone for a therapeutic process that is empowering, meaningful, and deeply personalized. That’s where real recovery begins.
Measuring Upper Extremity Use
When we think about evaluation in the context of neuroplasticity, primarily through the lens of learned nonuse, one of the most challenging questions becomes: How do we measure upper extremity use, especially in patients with lower motor function following brain injury?
The truth is, many traditional clinical practice assessments don’t capture this well. Tools like the Fugl-Meyer provide a useful overview of gross motor function. Still, when patients are too impaired to perform tasks like the Box and Blocks Test or the Nine-Hole Peg Test, or they’re not yet functional enough for assessments like the AMAT (Arm Motor Ability Test) or the ARAT (Action Research Arm Test), we’re left without great options.
So, how do we measure actual use of the impaired upper limb in meaningful daily life? In my clinic, I’ve found that self-report tools are an effective and insightful alternative. To gain a more comprehensive picture, I paired each self-report assessment with input from the caregiver. This dual perspective often reveals discrepancies between what the patient believes they’re doing and what others observe—a critical insight when addressing learned nonuse.
Here are the two tools I rely on most:
Motor Activity Log (MAL):
This more detailed self-report assessment evaluates how often and how well the patient uses their affected upper extremity in daily tasks. It includes common functional actions like turning a faucet, opening a door, pulling open drawers, and managing car doors. Each activity is scored on two scales: one for frequency and one for quality of movement. It’s a bit lengthy, so I often send it home with patients and ask them to bring it back completed. Even though it takes time, the results are incredibly valuable for setting goals and tracking progress.REACH Scale (Rating of Everyday Arm-use in the Community and Home):
This shorter and more user-friendly tool makes it a good alternative or complement to the MAL. One of its standout features is that it offers separate versions for dominant and non-dominant upper extremities. This matters because many patients, particularly those with left hemiparesis and dominant right hand use, will honestly say, “I wouldn’t have used my left hand for that task anyway.” The REACH tool accounts for that, offering a more tailored perspective on perceived use that factors in hand dominance.
Both tools can—and should—be integrated into goal setting. For example, I might write a goal like:
"Client will increase integration of the affected upper extremity in daily activities from a REACH score of 2 to a 4 over 4 weeks,"
or
"Client will improve frequency of use of the left UE for functional tasks, as measured by a 15-point increase on the Motor Activity Log."
These measures give us a quantifiable way to assess meaningful use, even when traditional tools fall short. They also support evidence-based practice, tie directly into neuroplasticity principles, and offer patient-centered ways to track progress.
Best of all, the Motor Activity Log and the REACH Scale are free online. You can typically find them on the Rehab Measures Database, making them easy to access and implement right away. They’re powerful tools for measurement and motivation, and I encourage you to begin incorporating them—especially for those patients who may otherwise fall through the cracks of conventional assessment tools.
Aerobic Exercise and Neuroplasticity
If you're using resources like the Rehab Measures Database, you're already taking a step toward evidence-informed practice. Now, let’s bridge that into a critical and evolving area of discussion in neurorehabilitation: aerobic exercise and neuroplasticity, primarily through the lens of intensity.
The concept of intensity is everywhere right now, whether you're hearing about it through continuing education, research literature, or social media. Terms like high-intensity circuit training (HICT), high-intensity interval training (HIIT), and high-intensity gait training are becoming common in neuro rehab discourse. But as this field grows, so do the debates. There are distinct “camps” of opinion forming around what we know, what remains unclear, and what should be adopted as best practice.
Let’s focus first on what we do know:
Moderate to vigorous aerobic exercise has been shown to increase brain-derived neurotrophic factor (BDNF), a protein that supports the growth, survival, and differentiation of neurons.
The research tells us that 30 minutes of aerobic exercise, three times a week, for at least four weeks, can lead to measurable changes in BDNF levels and positively influence functional recovery and neuroplasticity.
These findings are consistent across various populations, including stroke, traumatic brain injury, and even neurodegenerative disorders. So yes, aerobic activity matters, and intensity matters.
But—and here’s where it gets tricky—we don’t yet have a clear dose-response relationship. We don’t know exactly how much intensity is optimal for every patient, or how long or how often that intensity must be sustained to elicit consistent neuroplastic change. The current data gives us a solid foundation, but there’s still a lot of nuance.
Another challenge lies in how we define and measure intensity. In a research lab or high-tech rehab setting, clinicians may have access to heart rate monitors, VO2 max assessments, or lactate threshold testing. But most of us in day-to-day clinical practice don’t have access to those tools. So we need to find practical, clinic-friendly ways to gauge intensity.
Some options include:
The Borg Rate of Perceived Exertion (RPE) Scale: An excellent low-tech tool that asks clients to rate how hard they feel they’re working on a scale of 6 to 20 or using a simplified 0–10 version. Moderate to vigorous intensity typically falls between 12–16 (or 4–7 on the 0–10 scale).
The Talk Test: If your patient can still talk but not sing during activity, they’re likely working at moderate intensity. If they can barely talk at all, they may be in vigorous territory.
Observation and client feedback: Look for sweating, changes in breathing rate, facial flushing, and fatigue. Pair those with subjective reports.
So, while you might not have high-end equipment, you do have tools to help guide intensity safely and effectively.
The takeaway is this: aerobic exercise, done at the right intensity and frequency, primes the brain for change. It sets the stage for neuroplasticity and enhances outcomes from your functional, task-specific interventions. Start integrating short bursts of moderate to vigorous aerobic activity when appropriate for your patients—whether that’s through seated marching, fast-paced walking, biking, or dancing in place.
You don’t need a treadmill and a heart rate monitor to make it count. What matters is that we get people moving with purpose and challenge, and start treating aerobic intensity not as a bonus but as a core component of neurorehabilitation.
Intensity Matters
If you're using resources like the Rehab Measures Database, you're already practicing in an evidence-informed way. Now the challenge becomes translating that knowledge into real-time, practical decisions—especially around aerobic exercise and neuroplasticity. The topic of intensity has become a focal point in the field, and with good reason. We’re hearing more and more about high-intensity circuit training, high-intensity interval training, and high-intensity gait training. These ideas are gaining traction, but they're also generating a lot of debate, particularly around how we define intensity, how we measure it, and how we apply it safely in diverse clinical settings.
We know that moderate to vigorous aerobic exercise—30 minutes a day, three times a week, for at least four weeks—has been shown to increase brain-derived neurotrophic factor (BDNF), which promotes neuroplasticity. The evidence tells us that aerobic exercise is important and that intensity plays a key role in stimulating meaningful brain change. What remains unclear is the exact dose-response relationship. We don’t yet know how much intensity is required for each individual, or how to precisely tailor it across conditions and stages of recovery. This creates a practical challenge for therapists, especially in environments where patients may be medically fragile or cardiovascularly unstable.
In settings like the ICU, skilled nursing, or long-term acute care hospitals, pushing patients to their heart rate max may not be feasible or safe. So the question becomes: how do we still engage in intensity without compromising safety? The key is to get creative with how we measure and dose that intensity. One of the simplest and most accessible tools is the Borg Rate of Perceived Exertion scale. It allows us to gauge how hard patients feel they are working without the need for heart rate monitors. In addition, we can observe for signs like sweating, increased respiratory rate, or muscle fatigue. If a patient isn’t showing any of these signs—if they’re not sore the next day or breathing harder or showing effort—then we have to ask ourselves if we’re really challenging them enough. Sitting in a wheelchair and performing long arc quads or pedaling a bike passively doesn’t create the neurophysiological demand for plastic change.
We must consider increasing the challenge even in low-tech or low-resource settings. Active rest breaks can be one strategy. Consider whether a patient can stand or perch instead of letting a patient sit between exercises. If sitting, can they engage in a light cognitive or reaching task to maintain heart rate and mental engagement? When patients say they need to sit down, take the time to explore why. Are they genuinely fatigued? Are they losing their balance? Or are they bored and disengaged? Extending the time between rests and making those breaks more active can go a long way in increasing total therapy intensity.
We can also modify tasks to add a challenge. If a patient is practicing standing at the kitchen counter, add a resistance band to the refrigerator door or place items on unstable surfaces. Ask them to perform the same task in dim lighting or while doing a dual task. These complexity layers challenge motor skills and engage attention, working memory, and executive function. High-intensity interval training or circuit training is another way to add both cognitive and physical demands. In circuit training, patients move through different stations—maybe ten sit-to-stands, followed by a visual perceptual task, followed by resisted reaching, followed by a coordination or balance station. The constant switching builds alternating attention, increases overall cardiovascular demand, and keeps therapy dynamic. Almost anything can be turned into a circuit if we keep the patient moving and vary the task demands.
The bottom line is this: if you’re bored during a session, your patient probably is too. That’s a sign that the level of challenge isn’t high enough. Neuroplasticity requires novelty, salience, and effort. Therapy must be engaging and difficult enough to stimulate change, but safe enough to prevent harm. It’s not about having high-tech tools—it’s about using what you have with intentionality and creativity to keep patients moving, thinking, and adapting. That's where the true power of therapy lies.
Resources
I wanted to share a few quick resources to support your understanding and application of evidence-based intensity in neurorehabilitation. AOTA’s Choosing Wisely initiative includes some excellent material on evidence translation and how to apply intensity in a clinical setting. It's a helpful guide when looking for ways to align your interventions with best practices.
The Neuro APTA website is another great resource. It has a section called “Intensity Matters” and another titled “Evidence Elevates,” both of which include solid tools and references. They’ve compiled some great tables and detailed information on calculating and interpreting heart rate, training zones, and heart rate maximums. If you're newer to that kind of monitoring or need a refresher, their material lays it out in an accessible and practical way.
The best part is that you don’t need to be a member of AOTA or APTA to access these links. They're open to the public, so you should be able to explore and use all the content freely. These are solid resources as you integrate more intensity-based strategies into your therapy practice.
Intervention Ideas
Top Preferred Activities
In the research on patient preferences in occupational therapy, I thought seeing what activities patients prioritize was especially valuable. When asked about preferred activities, many patients identified tasks such as dressing, completing small daily chores, personal hygiene, eating, and transfers. These functional goals feel most urgent and meaningful to them, which makes perfect sense when you consider how essential these actions are to daily independence and quality of life.
This information becomes vital when considering how to approach upper extremity use in therapy. Our goals for upper limb rehabilitation can vary widely depending on the individual, but if we want to drive engagement and achieve outcomes that matter to our clients, we need to start with what they care about. A patient who wants to button their shirt or feed themselves without assistance will approach therapy differently than one working toward cooking or returning to a leisure activity. Starting with these high-priority ADLs helps ensure that the therapy is clinically relevant and personally meaningful, which, in turn, supports neuroplasticity, motivation, and follow-through.
Low-Level UE Use
When working with patients who have very low-level upper extremity use—those who are truly flaccid—it becomes essential to approach therapy from both a practical and protective perspective. Encouraging weight bearing is one of the most accessible and beneficial strategies for these individuals, but it has to be done with great attention to shoulder integrity. We always want to be mindful of shoulder subluxation, pain, and potential injury when weight bearing is introduced.
Recently, some skepticism has been around weight bearing, primarily tied to the shifting opinions about NDT approaches. While it's true that NDT as a formal framework has mixed evidence behind it, weight bearing itself is supported for several reasons outside that historical lens. It's not just about improving posture or alignment; weight bearing is vital in increasing bone density, building proximal muscle strength, and promoting somatosensory awareness. When a limb is flaccid or spastic and otherwise ignored or neglected, placing intentional weight through it creates both ascending and descending neural input that reinforces its presence and potential use in the body. There is real benefit to that kind of neural engagement.
For these lower-functioning patients, bilateral tasks are beneficial, even if the impaired arm only plays a helper role. For instance, bringing the affected arm to support a cup while the unimpaired hand brings the cup to the mouth, or allowing it to stabilize a bowl while eating. Even tasks like holding a pretzel rod or something similar—if the patient has any grasp, even a spastic grasp—can facilitate movement toward the mouth. It’s not perfect, but it’s practice, and practice builds awareness. Using a wash mitt is another creative idea. If the affected hand can’t manipulate a washcloth, placing the mitt over it allows the patient to engage in a basic self-care task like washing the opposite arm or face. You can even make one by folding a washcloth and sewing it into a simple sleeve. It may not seem like much, but those small moments of integration matter.
Another key strategy is to use the upper extremity for stabilization. For example, using the affected arm to hold an object against the table or trunk while the unimpaired hand completes a task, or bracing something between the hand and body during dressing or grooming. The overarching goal is not isolated movement but integration, creating functional ways to bring the affected arm back into participation. We’re not always trying to regain full grasp and release; we’re trying to help that arm become a functional assistant.
For home programming, I focus on real-world applications. My favorite go-to home program for these patients is elementary and functional: doorknobs, faucets, and light switches. These items are present all day, every day, in nearly every room of the house. Patients without fine motor control can often reach and interact with a lever-type faucet or light switch. The repetition is built into the routine. They can use the affected limb whenever they turn on a light, wash their hands, or open a door. This taps into repetition, relevance, and function all at once.
It’s easy for patients to remember, and it reinforces the principle that integration is the real goal—helping the affected limb find its way back into the daily rhythm of life, even if in a supporting role. Repeated throughout the day, that kind of use can create a foundation for larger gains over time.
Intermediate Level UE Use
For those intermediate-level upper extremity patients, the guiding principle remains clear: integration is everything. If they don’t use the limb, they risk losing the functional gains they’ve made. So, any activity that encourages consistent, purposeful use of the affected arm becomes valuable. Whether wringing out washcloths, folding towels, turning pages, or eating finger foods, these everyday tasks are perfect opportunities to reinforce engagement. The more meaningful and personally relevant the activity, the more effective it will be.
One patient I worked with had five children, including a baby. She didn’t have much distal control in the affected arm, but we found ways to use it during playtime with her infant. We focused on simple actions like pressing the buttons on baby toys or helping stabilize objects during play. These seem like small tasks, but they were powerful motivators because they tied directly into her identity and her roles at home. The limb wasn’t fully functional, but it was participating—and that matters.
When working with intermediate-level use, we also start to think about refining grasp, particularly by introducing lateral pinch. That might mean using the affected hand to stabilize the bottom of a zipper while the other hand pulls, or holding an envelope steady while the unimpaired hand slides something inside. These activities activate that initial grip function and promote more awareness and control, even in a limited range. Focusing on the ulnar side of the hand while maintaining wrist extension can help support the mechanics of grasp. If the patient is capable, we can begin working with tenodesis to leverage what functional movement is present.
Adaptations can be essential in this phase, and we shouldn't hesitate to use them creatively. I found Dycem to be a lifesaver after surgery on my arm, especially when I realized I couldn’t open pill bottles one-handed. Sometimes, the simplest, most affordable solutions from everyday stores can be just as effective as high-end adaptive tools. We don’t need to restrict ourselves to equipment from the clinic supply closet. We can also think outside the box by using household items from places like Walmart, the dollar store, or anywhere else we can source affordable, accessible tools to modify tasks and promote function.
The ultimate goal is to make these tasks achievable, meaningful, and part of the patient’s regular day, not something that happens only during a therapy session. By embedding these strategies into their daily routines and providing creative adaptations, we give them a better shot at functional recovery and long-term engagement.
High-Level UE Use
Then, those patients with high-level upper extremity use are the patients we're talking about—what do they want to do? How can we make things harder? This is the stage where we’re moving beyond just integration and beginning to isolate and strengthen movements while also gradually removing those adaptations they may have used earlier in their recovery.
We’re working on finger isolation and in-hand manipulation skills. So it’s time to go back to our pediatric friends, who really are the kings and queens of in-hand manipulation, and borrow from those techniques. Encouraging those fine motor skills—rotating, shifting, translating objects within the hand—is incredibly beneficial for this group. We’re also looking at increasing bilateral integration. At this point, it’s not just about getting the hand involved—it’s about getting both hands working together in more refined and complex ways. Even something as simple as thumb wrestling can be an awesome, engaging tool for strengthening and coordination.
So you can see how our approach in the clinic really differs based on the patient's level of upper extremity use. Whether they’re just beginning to engage that arm or trying to regain full functional control, our approach has to adapt accordingly.
Mental Practice
So, keeping things moving, let’s discuss mental practice as another method for driving neuroplasticity. I’ll move through this quickly, but it’s an exciting area, especially given its origins. Mental practice comes from sports psychology, and it's been studied extensively in elite athletes. Think about someone like Michael Phelps. Before a major swim event, he’d often be seen sitting on the pool's edge in a folding chair, headphones on, eyes closed. But he wasn’t just zoning out or listening to music for relaxation—he was engaging in mental practice. He was listening to a guided visualization script, mentally rehearsing every stroke of the race in perfect sequence, using multisensory cues to imagine how it would feel, sound, and look.
This same technique has been translated into rehabilitation. It’s something we can use with our patients, and it’s also something they can continue at home on their own. What the research shows is that mental practice can lead to improvements in motor function and even muscle strength. However, the evidence is more mixed when it comes to translating those gains into improvements in ADL performance. So while it’s not a complete substitute for physical practice, it can be a valuable complement, especially when movement is limited or additional cognitive engagement is needed.
There are structured ways to use mental practice in therapy sessions, and resources are available to help guide those sessions. I wanted to ensure you had access to that, so I’ve included a set of free downloadable mental practice scripts you can use in your clinic. These can be a great tool to help patients rehearse movements they’re working on and reinforce the neural pathways associated with successful task performance. It's a simple addition to therapy that can make a meaningful difference.
Action Observation
Action observation is another tool we can use to support motor recovery. This approach involves having the patient watch someone else perform a task while simultaneously doing it themselves. It’s based on activating the mirror neuron system, which is believed to play a role in motor learning by helping the brain simulate the movement internally as it's being observed.
We know from research that action observation can increase cortical representations and stimulate central nervous system activity through this mirror neuron activation. It’s been shown to help improve dexterity and may have some impact on reducing spasticity. However, it doesn’t appear to contribute much to muscle strength directly, and there’s mixed evidence regarding its overall effect on motor function and especially on activities of daily living.
So while action observation may not fully generalize to ADL performance, it can be a valuable tool for reinforcing foundational motor skills. It can lay the groundwork for further skill development and be paired with more functional ADL practice to bridge the gap from skill acquisition to real-world application.
Mirror Therapy
Mirror therapy is another excellent option to consider in upper extremity rehabilitation. In this approach, we place a mirror at the body's midline so that it reflects the movements of the non-paretic limb, creating the illusion that the affected limb is moving normally. This visual feedback is powerful—it activates the premotor cortex and engages the mirror neuron system, which can help stimulate neural reorganization and recovery.
We know from the research that mirror therapy can lead to improvements in motor function, dexterity, and proprioception. It allows the brain to “see” movement in the impaired limb, even if that movement isn’t physically occurring. That visual input can enhance the motor planning and sensory awareness processes. However, the effects are more mixed when translating those gains to activities of daily living, reducing spasticity, or building muscle strength. So, it’s not a standalone fix, but it can be a valuable part of a comprehensive program.
To give you a sense of how it works in practice, there’s a short video showing what this setup might look like with a patient. It’s a simple technique, often using just a basic mirror box, but the visual and neurological impact can be significant when used consistently and strategically.
Mirror therapy involves obstructing the view of the affected limb while positioning a mirror to reflect the unaffected limb. When the patient looks into the mirror, their brain perceives the reflected image as if the affected limb is moving. This illusion helps drive motor function by stimulating the areas of the brain responsible for movement and coordination. The visual input tricks the brain into believing that the affected limb is functioning normally, which can support the recovery process through neuroplasticity.
This technique engages the premotor cortex and activates the mirror neuron system, reinforcing motor pathways that may have been disrupted due to stroke or injury. It's a powerful tool because it gives the brain a compelling visual cue, which may encourage motor relearning and increased attention to the impaired side.
To support your use of this approach in the clinic, I’ve also pulled together some resources that outline how to structure a mirror therapy session, including what a typical session might look like and a sample script you can use to guide patients through the process. These tools can be a helpful starting point, especially if you want to integrate mirror therapy more intentionally into your treatment planning. It’s one more way we can harness neuroplasticity to promote function and engagement in recovery.
Motor exercises without an object | Motor exercises with an object |
Unilateral movements of the non-affected arm only | Unilateral movements of the non-affected arm with an object |
Bilateral movements (“as good as possible”) | Bilateral movements with an object only in the non-affected side |
Guiding of the affected arm by the therapist | Bilateral movements without objects on both sides (imagining the objects) |
Guiding of both arms by the therapist | Bilateral movements with guidance of the affected arm by the therapist (with or without an object at the affected side) |
I have also included a sample script that you might use with mirror therapy to make it available in your clinics and other tools that support neuroplasticity and can be applied in the clinical setting.
Watch the mirror as you complete the activities. Make sure you are trying to do these activities with your affected (right / left) hand at the same time. Do these exercises a minimum of 30 minutes per day, 5 days per week. Go slowly!
- Make a fist and then open your hand fully. Repeat 15 times.
- Pretend to play the piano, pushing each finger on the table one at a time. Continue for 2 minutes.
- Touch your thumb to the tip of each finger. Repeat 15 times for each finger.
- Place a washcloth on the table. Wipe the table in a circular motion, back and forth, and up and down, for 2 minutes.
- Place a water bottle on the table. Grasp it with your hand, lift it up 2 inches, place it back on the table and then let go. Repeat 15 times.
- Place 5 coins on the table. Pick them up one at a time until they are all in your palm. Place them back on the table, one at a time, using your thumb with your index and middle fingertips.
Repeat the entire process 3 times.
ADL Moments
- Toileting
- AM ADL routine
- Showers/bathing
- Self feeding
- Simple home management in the patient’s room (making bed, hanging up clothing)
You guys, don't be afraid to use those ADL moments—especially those of you working in skilled nursing facilities, inpatient rehab, or acute care settings. If a patient needs to go to the bathroom, I think, great, let’s go—this is our opportunity to work on functional mobility. If I happen to be there when their meal tray arrives, that’s a perfect time to practice self-feeding. Can we make the bed together? Can we hang up clothes in the closet? Whatever the situation is, it’s about recognizing and using those real-time ADL moments as meaningful therapeutic opportunities.
Join Activities
- Check the activities calendar for opportunity
- Bingo for cognition and visual scanning
- Balloon volleyball
- Crafts
- Therapy in this setting can provide not only intervention, but also social interaction.
And then those of you that work in facilities, again, activities are a great thing.
There's lots of things we can do in activities. It increases social interaction as well. But also if you have those patients that are refusers, this is a great way to get them doing therapy. I wanted to give you guys ideas of some things
Holidays
- Fill Easter eggs for the facility hunt (or help hide them)
- Decorate the Christmas tree or hang decorations
- Plan a holiday party
- Make gifts or cards for loved ones
they could do just to integrate in the holidays, again, skilled nursing facilities, outpatient clinics, lots of things to do. And a big thing is making gifts or cards for loved ones because these patients aren't able to go and purchase something.
So they love to have something to give around. A birthday or a holiday.
Involve Leisure Interests
- Putting for golfers
- Knitting/Crochet
- Card games
- Painting
- Models
- Puzzles
- Gardening
Lots of things with leisure interests we can pull in that are meaningful and use this motor framework. So tons of things we can do with this
Homemaking Tasks
- Folding or sorting laundry
- Making the bed
- Cooking/baking tasks
- Sweeping
- Dusting
- Sorting silverware into dividers
and the same thing with homemaking. So again, wanted to give you guys a lot of resources for just some ideas to spark your brain.
But we're not going to spend a ton of time on these slides because these are just things to spark your brain to get you going with some ideas.
Vocational Tasks
- Making copies or packets of papers
- Crushing aluminum cans
- Helping with inventory
- Clean out a closet or cupboard
- Wipe down therapy equipment
- Multiple errands tasks
Some ideas of work tasks here for you. Wiping down therapy equipment, helping with inventories, all of those types of things.
Ice Cream in a Bag
http://spoonful.com/recipes/homemade-ice-cream-bag
I wanted to share this idea for ice cream in a bag, which works a lot of hand strength,
Grip and Pinch Strength
- Make paper wads (and throw them into trash)
- Knead Dough
- Aggression Cookies
- http://allrecipes.com/recipe/aggression--oatmeal--cookies/
- Pie Crust
a lot of hand activity and some ideas for grip and pinch strength. And again, this idea for aggression cookies is just a research recipe.
Here you put everything on a tablecloth and you just knead it with your hands. It's a really great recipe for patients that maybe we're just needing to get some of that hand strength and intrinsic muscle strength.
But the bottom line of all of this is whatever you do, we just want to make it functional. We want to pull in function right quickly.
High Tech Interventions: Virtual Reality
We're going to go through some high tech interventions as well.
These are things we're not going to spend a lot of time on because a lot of you aren't going to have access to these things in the clinic. But I do want to touch on them because they're really coming down a lot in the research. So this idea of virtual reality, I know some of our clinics are getting VR equipment. What the research shows us is it may not be more beneficial than conventional therapy for promoting or improving motor function and stroke severity. And there's really no evidence for improvement in ADL's dexterity and spasticity or muscle strength.
High Tech Interventions: Transcranial Direct Current Stimulation
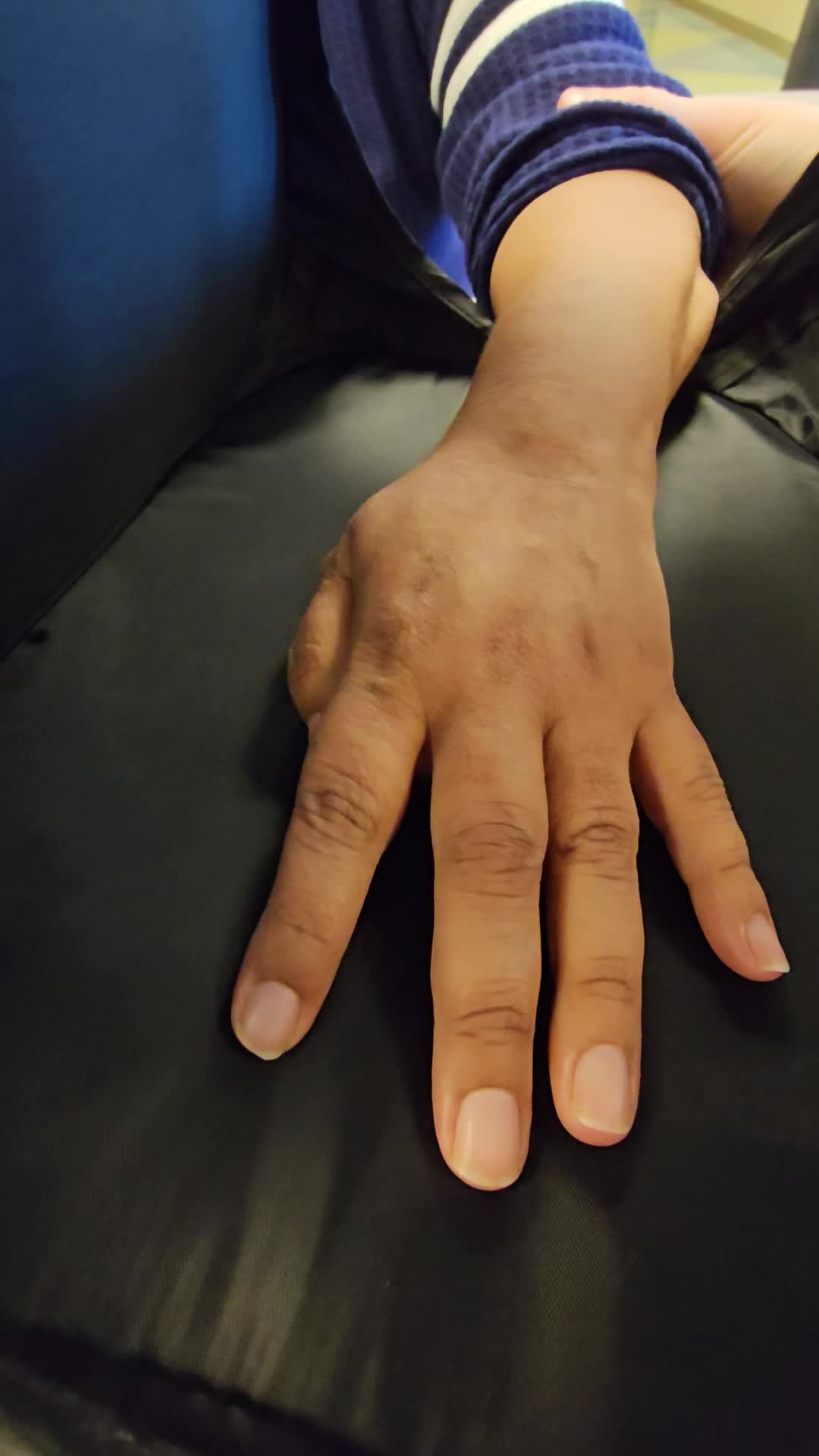
So while this may be another way to get the reps and to engage a patient, there's really not a ton of benefit research wise over what we're doing in the clinic. Conventionally, this is just a video of transcranial direct current stimulation. So this is something that you need additional training to do. But this is a video of the gentleman prior. So same session we're trying to get supination.


We're really struggling there. Right. And then we added in transcranial direct current stimulation, which is really e stim like using a, like an iontophoresis machine or a TCDS machine and it directly through the skull into the brain. So you can see with that transcranial direct current stem, we're still not getting great results, but we're getting better results with this. Right.
So this is something that is coming down in the Research with all of the neuromodulation studies, you know, it kind of has mixed evidence. So you can do anodal cathodal or dual transcranial direct stimulation, which just means it's just a fancy way to say where you put the positive and negative. But there's mixed evidence for it alone or in combination with other approaches. The other thing with this is we know sometimes that it helps patients in the immediate, but we don't know what the potentiality of this is. Right.
We don't know how long this lasts. So we're still doing a ton of research on these items. While transcranial direct current stim is subthreshold, so we're not getting a motor movement from it.
Transcranial Magnetic Stimulation
The idea of transcranial magnetic stimulation, which is an electromagnet, is supra-threshold. So, they use functional MRI mapping, which you can see here, to figure out where to use this.
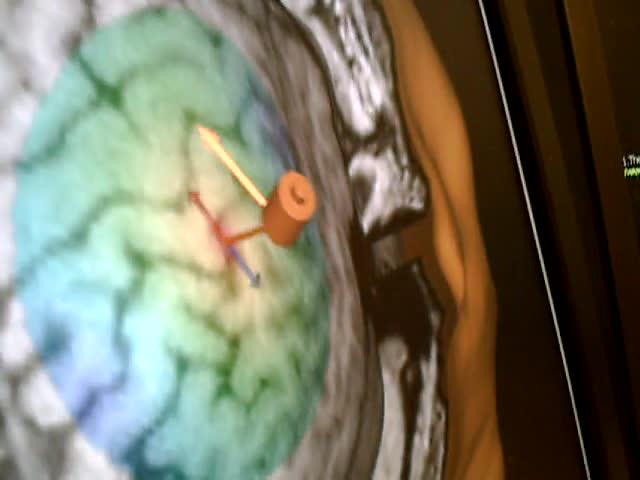
They use EEG mapping along with this, and it’s considered super-threshold. So, as the magnet activates with the patient, we see motor movement in the hand. This is very different from some of the other interventions we’ve discussed. You’ll sometimes see transcranial magnetic stimulation—TMS—used in large research centers. It’s also becoming more common in mental health settings in the form of repetitive TMS, or rTMS.
What we know about TMS is that low-frequency stimulation may be beneficial for improving motor function, dexterity, ADLs, proprioception, and reducing stroke severity. Still, it does not appear to impact spasticity or range of motion. On the other hand, high-frequency stimulation may support improvements in dexterity, ADLs, stroke severity, and muscle strength, but it hasn’t shown benefits for general motor function.
I just wanted to touch on this briefly because, while promising, these interventions require additional training and specialized equipment. They cannot typically be implemented without further preparation, but they’re good to be aware of as part of the expanding landscape of neurorehabilitation.
Summary
In conclusion, the key points to take away are that function truly matters, salience matters, and repetition, intensity, and challenge are really central to driving change. We want to hold onto and apply these essential elements in our day-to-day practice. With that in mind, let’s move into some of the questions.
Questions and Answers
I have a patient who had an initial stroke on the left side of the brain, and within a few days, they had a second stroke on the right side. It's been two months. They only have use of the right arm and hand, have a tracheostomy, and are unable to swallow. What is a realistic outcome?
That’s an excellent and complex question. Realistic outcomes can be challenging to predict in stroke patients, especially with bilateral involvement. However, there are a few clinical indicators we can reference. For instance, early return of finger extension (within 48 hours) is a strong prognostic indicator for better motor recovery. Without knowing more (e.g., vision, cognition, perceptual abilities), the most practical approach is to maximize use of the functional right upper extremity. We focus on ADL participation, compensatory strategies, and eventually prepare for discharge once we've reached a plateau. Keep in mind—this is a process of exploration and ongoing reassessment.
How long is it expected to take to see improvements in upper extremity use?
The answer is: Never. There's no such thing as “too long.” Neuroplasticity doesn’t have an expiration date. We see patients improve years after stroke—what drives change is repetition, habituation, and meaningful engagement. When we say "upper extremity use," this doesn't always mean fine motor skills like buttoning. It might be as simple as stabilizing paper, using the limb as a weight, or incorporating it into tasks as a functional assist.
How do we work with flaccid paralysis of the upper extremity to improve function and neuroplasticity?
Start by protecting and integrating the arm into the person’s visual and physical space. Many patients with flaccidity show inattention to the limb, so we emphasize line-of-sight placement, frequent handling, and stimulating the sensory pathways. Techniques include:
Positioning to prevent injury and promote awareness
Functional integration, even if passive at first
Neuromuscular electrical stimulation (NMES) to activate muscles
Neuromodulation, if available
Repetitive task practice and task-specific training
This helps reinforce cortical engagement and lays the groundwork for improved function.
References
See the additional handout.
Citation
Reimer, A. (2025). Changing the brain: Using the principles of neuroplasticity and motor learning to improve functional outcomes in acquired brain injury. OccupationalTherapy.com, Article 5798. Available at www.occupationaltherapy.com
