Editor's note: This text-based course is a transcript of the webinar, Occupational Therapy Practitioner's Role In The Cardiac ICU, presented by Brittany Rohrer, MS, OTR/L.
*Please also use the handout with this text course to supplement the material.
Learning Outcomes
- After this course, participants will be able to list common cardiac diagnoses and surgical interventions that may necessitate ICU level of care.
- After this course, participants will be able to recognize important clinical considerations for the occupational therapy practitioner in the cardiac ICU setting.
- After this course, participants will be able to identify OTP’s role in the assessment and treatment of specific patient populations in the cardiac ICU.
Benefits of Early Mobility/Activity
in the ICU
Before we dive into our main learning outcomes, I want to take a moment to discuss the benefits of early mobility and activity in the ICU, as supported by current research. One of the primary benefits is a reduction in intensive care unit-acquired weakness. This condition involves bilateral, symmetrical limb weakness and/or neuromuscular weakness. It typically develops within the first 10 days of a person’s ICU admission and can appear as early as day two. Early mobility helps to counteract this complication.
Additional benefits of initiating activity early in the ICU include enhanced cognitive and functional recovery, improved muscle strength, and a decrease in the incidence of delirium. Delirium is characterized by an acute decline in attention and awareness, and it unfortunately occurs in a high percentage of ICU patients. I've seen this firsthand in clinical practice, and the difference early intervention can make is significant.
Further positive outcomes associated with early mobility include a decreased duration of mechanical ventilation, shorter ICU and overall hospital stays, reduced mortality rates, and improved quality of life after ICU discharge. Early mobility also helps reduce the incidence and severity of post-intensive care syndrome, or PICS. PICS describes a set of impairments in physical function, mental health, and cognition that many patients experience after leaving the ICU.
As an occupational therapy practitioner, I recognize our critical role in promoting early mobility and activity and restoring function for our patients across all ICU settings. This is especially true in the cardiac ICU, which will be the focus of our discussion today. Our interventions are supportive and essential in driving recovery and improving outcomes for these vulnerable patients.
Heart Failure
First, I want to talk about heart failure, as many of our patients in the ICU may present with this diagnosis. Heart failure is a chronic, progressive condition in which the heart muscle is unable to pump enough blood to meet the body's metabolic needs. This means that vital organs and tissues may not receive the oxygen and nutrients they require, contributing to a range of clinical symptoms and challenges in care.
In patients with heart failure, we may observe several signs and symptoms. Dyspnea, or shortness of breath, is common and often worsens with activity or while lying flat. This difficulty breathing while lying down is known as orthopnea. Patients may also experience persistent coughing or wheezing, which can be distressing and interfere with rest and sleep. Edema is another hallmark sign and can appear in the extremities or abdomen as the body holds on to excess fluid. Alongside this, weight gain may occur rapidly due to fluid retention.
Fatigue is frequently reported and may be profound, limiting participation in even basic activities of daily living. Some patients lose their appetite or experience nausea, which complicates nutritional intake and overall recovery. Altered mental status may also be present and can range from mild confusion to more serious cognitive changes, which can be especially concerning in the ICU. Lastly, tachycardia, or a rapid heart rate, may be observed as the heart tries to compensate for its decreased efficiency.
Understanding these symptoms is essential for tailoring occupational therapy interventions in the ICU, ensuring we address both the physical and cognitive needs of patients with heart failure.
Stages
These are the stages of heart failure as per the American Heart Association.
Stage A: | At risk for heart failure but do not yet have symptoms or structural or functional heart disease
Risk factors include hypertension, coronary vascular disease, diabetes, obesity, exposure to cardiotoxic agents, genetic variants for cardiomyopathy, and family history of cardiomyopathy |
Stage B: | No current or previous symptoms of heart failure, but with either structural heart disease, increased filling pressures in the heart, or other risk factors |
Stage C: | Current or previous symptoms of heart failure |
Stage D: | Heart failure symptoms that interfere with daily occupations or lead to repeated hospitalizations |
The patients we typically see in the cardiac ICU are in the more advanced stages. stages C and D, where they have or have previously had symptoms, and these symptoms may interfere with their daily occupations or lead to repeated hospitalizations.
Classes
So, the American Heart Association classifies heart failure in patients in stages C and D according to the severity of their symptoms.
Class | Patient Symptoms |
I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation or shortness of breath. |
II | Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, shortness of breath or chest pain. |
III | Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, shortness of breath or chest pain. |
IV | Symptoms of heart failure at rest. Any physical activity causes further discomfort. |
Class I heart failure includes patients who experience no limitation in their physical activity. They can perform ordinary activities without any symptoms. In Class II, there is a slight limitation of physical activity. Patients may feel comfortable at rest, but ordinary physical activity can result in fatigue, palpitations, or dyspnea. A marked limitation in physical activity characterizes class III. Although patients are still comfortable at rest, even less-than-ordinary activities can lead to noticeable symptoms. Class IV is the most severe, where patients exhibit symptoms of heart failure even while at rest, and any physical activity causes additional discomfort.
When reviewing a patient's medical record, it’s common to see the physician refer to the patient’s ejection fraction. This key measurement is used to evaluate heart function and is typically expressed as a percentage. It indicates how much blood the left ventricle pumps out with each contraction. Understanding a patient’s ejection fraction helps guide our clinical reasoning and the activity level we might safely introduce in therapy, especially in the ICU, where patients can be medically fragile.
Ejection Fraction (EF)
This measurement, abbreviated as EF for ejection fraction, reflects how much blood the left ventricle pumps out with each contraction. A normal or preserved ejection fraction falls between 50 to 70% and is commonly noted in the medical record as HFpEF—heart failure with preserved ejection fraction. When the ejection fraction is between 41 to 49%, it's considered borderline. A reduced ejection fraction is defined as less than or equal to 40%, abbreviated as HFrEF—heart failure with reduced ejection fraction.
Patients may require admission to the cardiac ICU for several reasons related to heart failure. Some may be experiencing a new onset of heart failure, while others may be admitted due to an acute exacerbation of a chronic condition. These situations often require close monitoring, medication titration, and hemodynamic support.
Patients can also be admitted for treatment of secondary heart failure, which may stem from other cardiac events or conditions. This can include severe valve disease, cardiac arrest, arrhythmias such as an irregular heartbeat, or a myocardial infarction, which is a heart attack. Additionally, some patients enter the cardiac ICU as part of the evaluation process for advanced heart failure therapies. This can include consideration for a left ventricular assist device (LVAD) or evaluation for a heart transplant, both of which we will explore in more detail shortly.
OT Considerations
There are several important considerations when working with patients who have heart failure, especially in the ICU. One major factor is that many of these patients may be on one or more vasoactive drugs. These medications are used to support patients with severe hemodynamic instability by helping to restore adequate tissue perfusion. They include both inotropes and vasopressors, and some of the common medications you might see in a patient’s chart are dopamine, dobutamine, norepinephrine, epinephrine, and milrinone.
These medications can come with a range of potential side effects. Some patients may experience severe hypertension, or high blood pressure, while others may present with hypotension, or low blood pressure. Ventricular arrhythmias, which are irregular heart rhythms originating in the ventricles, are also a known risk. Cardiac ischemia and tissue ischemia can occur when there isn’t enough blood flow to the heart or other tissues. This can be particularly concerning in the extremities; I’ve seen patients whose digits became discolored, painful, and in some unfortunate cases, necrotic due to poor perfusion. Other side effects may include tachycardia, which is a fast heart rate, bradycardia, a slow heart rate, or even sudden cardiac death.
Despite these risks, it’s important to know that studies support the safety of therapy for patients on vasoactive drugs—as long as they have stable hemodynamics. The evidence shows a low incidence of adverse effects during therapy, and most of those events are not serious. That said, we as occupational therapy practitioners must always take a cautious approach. Slow, progressive mobilization is key, and we should maintain constant communication with the interdisciplinary team to ensure that we are aligning with the medical plan of care and prioritizing patient safety at all times.
In addition to medication management, general heart failure management strategies are essential to understand. Patients are typically educated to weigh themselves daily and record their weights. A weight gain of more than two to three pounds in one day, or more than five pounds in a week, is considered significant and can indicate fluid retention. These patients are usually placed on a low sodium diet, with less than 2300 milligrams of sodium per day, and they may also be on a fluid restriction to help prevent excess fluid buildup. These strategies, combined with medication adherence and lifestyle adjustments, are all part of a comprehensive plan to manage heart failure and prevent exacerbations. As therapists, being aware of and integrating this knowledge into our care helps us better support our patients in achieving safer and more meaningful outcomes.
OT Role
Now that we’ve explored the key considerations and background related to heart failure, let’s look at what our role as occupational therapy practitioners truly involves. Knowing our patients' complexities, particularly in the ICU setting, we can prioritize cognitive interventions that promote independence with heart failure management. For example, we can work with patients on developing routines for weighing themselves daily, helping them with the physical task of stepping up and down on the scale, and suggesting environmental strategies like keeping a notepad next to the scale so they can easily record their weights. This small step supports both habit formation and adherence to self-management.
We can also address blood pressure monitoring as part of therapy. If a family member is available, asking them to bring in the patient’s home blood pressure cuff is helpful. Practicing with the equipment they’ll use at home provides consistency and builds confidence. If that home cuff is inaccurate, we can teach the patient and their family how to compare readings with the hospital unit to understand better how much it may vary, which is an important consideration when monitoring their health post-discharge.
Our role also includes helping patients learn to manage their medications, keep track of medical appointments, and track fluid intake if they’re on a fluid restriction. We can support meal planning and strategy development for adhering to a low-sodium diet, which they must maintain after leaving the hospital. I provided a link to the Menu Task, which is a free performance-based screen that evaluates functional cognition. It includes prompts like “select two or more heart-healthy food items” or “do not exceed 1,800 total calories,” allowing us to assess the patient’s ability to follow restrictions and make appropriate food choices. This offers great insight into how well they might manage once they return home.
In addition, we can teach energy conservation techniques to help them manage fatigue during daily activities. This may include instruction on pursed lip breathing, pacing strategies, taking seated rest breaks, using proper body positioning during tasks, and integrating the Rate of Perceived Exertion (RPE) scale to gauge their effort level. We’ll talk more about that later. Goal setting and treatment planning can also be guided by tools like the Self-Care of Heart Failure Index, a self-assessment that evaluates maintenance behaviors, symptom management, and confidence. This tool can help flag areas where patients may struggle with compliance or follow-through once they leave the ICU.
Another central aspect of our role is facilitating safe early activity and mobility. We take a slow, graded approach while carefully monitoring vital signs, ensuring our interventions align with medical stability. Functional training is also key. We provide self-care retraining, task modifications, and compensatory strategies to address any impairments caused by the heart failure. For example, we may introduce adaptive equipment like a reacher to assist with lower body dressing and reduce the need for bending, or a universal cuff to aid with feeding and grooming if the patient has decreased hand function. This can be particularly helpful if they’ve experienced tissue ischemia or developed necrotic digits as a result of prolonged use of vasoactive drugs.
We also support patients in developing individualized therapeutic exercise programs. These may include range of motion, gentle strengthening, dexterity work, and stretching—all aimed at preserving function and promoting recovery.
Delirium prevention is another critical part of our practice, particularly in the cardiac ICU. Since delirium represents an acute change in a patient's attention and awareness, we can implement interventions to help normalize the sleep-wake cycle. This includes strategies like keeping lights on, window shades open during the day, and turning off lights and closing shades at night. Reorientation is significant—verbally cueing the patient to the current time, date, and place, while providing visual supports like a clock within view of the bed and a calendar in the room to reinforce this information.
We can also encourage cognitive stimulation during downtime. Activities such as reading the newspaper, doing crossword puzzles, listening to music instead of television, and engaging in hobbies they enjoyed before their hospitalization can all help maintain engagement and orientation. Involving family members in selecting these activities can make the experience more meaningful. Another key element is noise reduction, since research has shown that noise is one of the most significant sleep disruptors in the ICU. We can help by educating staff and families about protocols for maintaining quiet environments and promoting sleep hygiene.
Finally, it’s worth noting the impact of simple actions like assisting patients in donning their glasses or hearing aids before beginning any activity. These basic but essential tasks allow patients to use all of their senses and interact more effectively with their environment. Each of these interventions plays a role in optimizing recovery, promoting self-management, and enhancing the overall quality of life for our patients with heart failure.
Open Heart Surgery
The next topic we'll discuss is OT's role in treating individuals status post-open heart surgery. This includes those who have undergone any surgical intervention requiring median sternotomy to gain access to the heart. This is a cut right through the sternum or breastbone. The sternum is then closed with wire, taking about six weeks to heal.
Example: Coronary Artery Bypass Graft (CABG)
The first example is coronary artery bypass graft, or CABG (Figure 1).
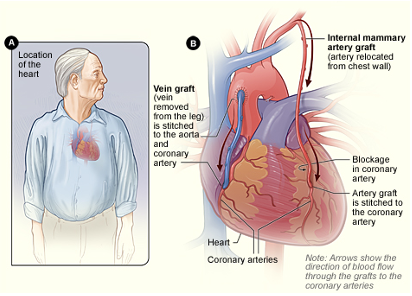
Figure 1. Graphic of a CABG.
This surgical procedure involves using a vein or artery from another part of the body to bypass a clogged coronary artery to restore adequate blood flow to the heart. This photo shows the internal mammary artery and a vein from the lower extremity being grafted to bypass two blocked coronary arteries. You'll see this abbreviated in the medical record as CABG times one, CABG times four, et cetera.
Example: Valve Replacement
Another example of a surgery that requires a median sternotomy is valve repair or replacement. This type of procedure is performed when there is severe malfunctioning of one or more of the heart’s four valves—either the aortic, mitral, pulmonary, or tricuspid valve. The underlying issues can include stenosis, the narrowing of the valve opening; regurgitation, where the valve does not close properly and allows blood to flow backward; or prolapse, where the valve flaps bulge or collapse backward into the chamber.
Figure 2 shows aortic valve regurgitation.
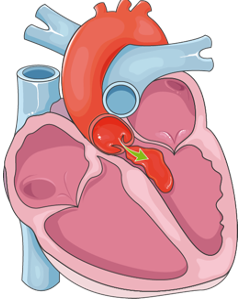
Figure 2. Aortic valve regurgitation.
Under normal circumstances, blood is pumped from the left ventricle into the aorta through the aortic valve. However, in this example, the aortic valve is not functioning properly, allowing blood to leak back into the left ventricle instead of moving through the circulatory system. This kind of backflow increases the workload on the heart and can lead to symptoms such as fatigue, shortness of breath, and fluid buildup. This condition often requires surgical intervention to restore normal valve function when left uncorrected.
Understanding the nature of these surgical interventions allows us as occupational therapy practitioners to tailor our approach appropriately. We play a vital role in educating patients on sternal precautions, essential for protecting the integrity of the healing sternum. We also help patients learn how to modify daily activities to reduce physical strain, and we support them in regaining independence while respecting the physical limitations of a healing chest wall.
This issue is typically addressed through either a valve repair or a valve replacement. In the medical record, repairs are often abbreviated using a lowercase "r" following the valve involved, such as "AVr" for an aortic valve repair. Conversely, replacements are documented with an uppercase "R," so "AVR" indicates an aortic valve replacement.
While there are instances in which surgeons can perform a transcatheter aortic valve replacement, or TAVR, a minimally invasive procedure accessed through a small incision in the groin, our focus here is specifically on valve replacement via median sternotomy. This surgical route requires a more involved recovery process and further emphasizes the importance of OT in postoperative care planning and intervention.
Example: Thoracic Aortic Aneurysm Repair
Another example includes a thoracic aortic aneurysm repair. This is the repair of an aneurysm or a weakening and bulging in the ascending aorta, aortic arch, or descending thoracic aorta.
Example: Type A and B Aortic Dissection Repair
Aortic dissection repairs of type A and type B may be performed. An aortic dissection is a tear in the lining of the ascending aorta, which is considered type A, or the descending aorta, which is considered type B.
Example: Heart Transplant
Figure 3 shows the last example.
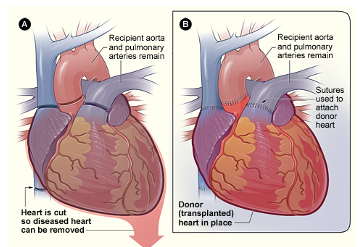
Figure 3. Heart transplant graphic.
This is a procedure where a failing heart is replaced with a healthier donor heart. So this is performed in advanced cases of heart failure where no other treatments have worked. There is a very extensive evaluation that goes on to determine whether a patient is even an appropriate candidate for a heart transplant. And once they are deemed a candidate, the patient is put on a wait list and essentially waits for a heart to be a good match.
OT Considerations
Some important occupational therapy considerations when treating patients status post open heart surgery include understanding and addressing sternal precautions. These precautions involve movement restrictions to protect the sternum while it heals during the first several weeks following surgery. While these precautions are standard in most postoperative protocols, they can vary significantly between institutions nationwide and worldwide. Typically, patients are instructed to avoid pushing, pulling, or lifting more than 10 pounds—the equivalent of a gallon of milk. There are also often restrictions on specific arm movements, and these precautions may be in place anywhere from 6 to 12 weeks, depending on the surgical team and the patient's recovery progress.
In recent years, new research has challenged the traditional approach to sternal precautions, offering alternative methods for protecting the healing sternum. One example is the “Keep Your Move in the Tube” method. This approach draws on kinesiology and ergonomic principles rather than relying on strict time or load limits. With this method, there are no hard lifting restrictions. Instead, the focus is on maintaining upper extremity movement within a safe "tube" close to the body, encouraging functional movement patterns that minimize strain. It’s important to note that to implement this method, institutions need formal approval to use the associated materials, and they must commit to adopting all aspects of the approach as outlined by its developers.
For OT practitioners, engaging in open conversations with the cardiac surgery team about their expectations and practices regarding sternal precautions is perfectly appropriate. Suppose you and your therapy colleagues find an alternative approach, such as “Keep Your Move in the Tube,” that may benefit your patient population. In that case, these discussions can help guide institutional change and promote best practices. The key is to work collaboratively with the care team and stay informed about evolving evidence that can enhance patient outcomes.
There are other key considerations as well. After a CABG procedure, the site from which a graft was harvested may be sore and impact the patient’s function. For example, if a radial artery was used, the patient may have pain, reduced wrist range of motion, or decreased strength in that extremity. Suppose it happens to be their dominant hand. In that case, this can have a notable effect on daily activities, and we may need to incorporate specific interventions to support their independence and comfort.
Institution-specific practices also influence our role. Different hospitals may have varying expected lengths of stay in the cardiac ICU and other protocols related to recovery timelines. For example, some institutions require patients to shower before discharge, and cardiac surgeons may want this initiated while the patient is still in the ICU. Depending on the setting, occupational therapists may be actively involved in showering the patient, or we may focus more on education about proper showering techniques and incision care.
Another essential detail to consider, especially when working with female patients, is the use of surgical bras. These are typically front-opening and secured with Velcro, designed to provide gentle, external support to minimize pulling or tension on the healing sternum. We often assist patients with donning and doffing these surgical bras as part of their self-care training.
Understanding and navigating these variables allows us to provide meaningful, individualized care that supports the patient’s recovery while promoting safety, confidence, and functional independence.
Borg Rating of Perceived Exertion (RPE) Scale
Another interesting fact to remember when working with heart transplant recipients is that their heart rate does not reliably reflect their actual activity intensity. This is because after transplantation, the heart is denervated, meaning it no longer receives autonomic nervous system signals in the same way. As a result, there is a delayed heart rate response to exercise, which makes it challenging to use heart rate as a dependable measure of exertion.
In these cases, we often rely on the Borg Rating of Perceived Exertion (RPE) scale to help the patient and the therapist gauge how hard the body works. This scale allows us to determine whether we should continue a given activity, adjust the intensity, or stop altogether. For patients who have undergone a heart transplant, therapy is generally targeted to an RPE level between 11 and 14 on the Borg scale. This range is described as “light” to “somewhat hard,” providing a safe and manageable level of exertion for individuals still recovering from major surgery.
It's also important to incorporate a warm-up and a cool-down phase in any exercise session for these patients. These preparatory and recovery periods are critical given the altered physiological response of the transplanted heart. Using the Borg scale helps ensure we're not overexerting the patient while promoting progressive functional gains. In the visual aid referenced, the relevant section of the Borg scale is highlighted to emphasize the target range for guiding therapeutic activity post-transplant.

Figure 4. Borg Rating of Perceived Exertion (RPE) Scale.
This is the modified Borg scale in Figure 5, which may be a little easier for patients to use because it's just a zero through 10 scale.
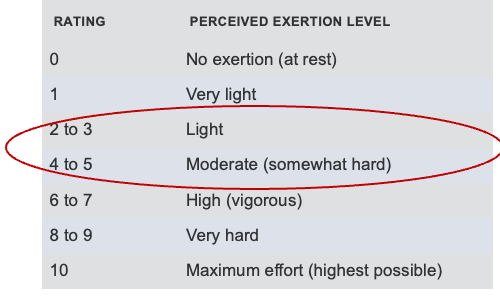
Figure 5. Modified Borg Rating of Perceived Exertion (RPE) Scale.
Again, you want your patient to feel like they're working in the light to moderate areas. If they start feeling like the activity is too hard, then we'll encourage them to stop and take a rest.
OT Role
The role of the occupational therapy practitioner in treating patients after open heart surgery is multifaceted and centers on facilitating safe, early activity and mobility. As mentioned earlier, this mobilization should follow a slow and carefully monitored progression while continuously observing the patient’s vital signs. In many cases, patients may be expected to begin getting out of bed as early as post-operative day zero, the day of surgery. However, OT involvement typically begins around post-op day one. At that point, we might complete the initial evaluation and introduce very light, guided activity to promote recovery without compromising the healing process.
A key part of our intervention is providing self-care training that incorporates task modifications to ensure adherence to sternal precautions. We collaborate closely with nursing, physical therapy, and the broader interdisciplinary team to coordinate care and ensure safety. Reinforcing energy conservation techniques is equally important, particularly those strategies we previously discussed in the context of heart failure. These techniques are just as relevant post-surgery, as patients often face similar fatigue levels and decreased endurance.
Pain management is another critical component of care. We must coordinate with nursing staff to ensure patients receive appropriate pain medication before therapy sessions so they can participate comfortably and effectively. Positioning after therapy is also important. Whether the patient is in bed, in a chair, or a wheelchair, we want to provide proper support under the arms and ensure that the chest is adequately supported to avoid strain on the healing sternum.
We can also help develop individualized therapeutic exercise programs, which may include postural exercises like gentle shoulder shrugs and circles. It’s important to discourage patients from overusing the heart pillow typically provided after surgery. While this pillow is valuable for delivering counter pressure during coughing or sneezing to reduce sternal pain, some patients become overly reliant on it and use it as a crutch, which can promote a rounded posture. Our goal is to help patients maintain an open chest posture and avoid patterns that may delay recovery.
In addition to postural work, we may incorporate active range of motion and strengthening exercises, as well as promote the use of the incentive spirometer. This handheld device helps patients practice deep breathing, and while it is usually introduced by nursing or respiratory therapy, we play a vital role in reinforcing its use. We can remind patients to complete their breathing exercises consistently—typically around 10 repetitions every hour while awake—and encourage them to continue using the spirometer after discharge from the cardiac ICU.
Other aspects of our role include reinforcing heart failure self-management strategies when appropriate and implementing delirium prevention interventions. Medication management is also a significant focus, particularly for patients who have undergone a heart transplant. These patients will be prescribed potent immunosuppressant medications to prevent organ rejection, and these drugs often come with hazardous drug precautions. We need to be aware of our institution’s protocols for handling such cases, which may include measures like double-gloving when managing bodily fluids. Understanding these precautions ensures that we can protect ourselves and our patients while continuing to support their recovery in a safe and effective manner.
Left Ventricular Assistive Device (LVAD)
Now, we’re discussing occupational therapy's role in treating patients status post LVAD implantation in the cardiac ICU. LVAD stands for Left Ventricular Assist Device, a mechanical circulatory support system used to treat patients with end-stage heart failure. It functions as an implantable blood pump that diverts blood from the left ventricle and delivers it into the aorta, helping maintain adequate cardiac output when the heart can no longer do so effectively.
LVADs may be used for different purposes depending on the patient’s overall prognosis and treatment plan. Some patients receive an LVAD as a bridge to transplant, meaning it supports them while they await a heart transplant. Others may receive it as destination therapy, which means the LVAD is a permanent solution, and they will live with it for the remainder of their life. In some rare instances, an LVAD is used as a bridge to recovery, where the heart regains enough function over time that the device can eventually be explanted.
There are several types of LVADs, but we primarily focus on the HeartMate 3 in our current practice. As of June 2021, the HeartWare HVAD system is no longer being implanted, although you may still encounter patients who have this device from a prior surgery.
The LVAD consists of an internal blood pump connected to an external controller via a driveline. This driveline exits the body through the abdomen and requires meticulous care to prevent infection. While the patient is in the hospital, this driveline site is covered by a sterile dressing that should only be changed by nursing staff. Once the patient is discharged, the responsibility for sterile dressing changes transitions to the patient and their caregiver, who are trained to manage it safely at home.
As occupational therapy practitioners, our role involves helping patients adjust to life with an LVAD by addressing the physical, cognitive, and emotional demands of living with this complex device. We promote safe mobility, modify self-care routines, support energy conservation strategies, and reinforce education related to the patient’s functional and home environment needs. Understanding the structure and function of the device—including components like the driveline and external controller—is essential to delivering effective and informed care in the cardiac ICU and beyond. An example is in Figure 6.
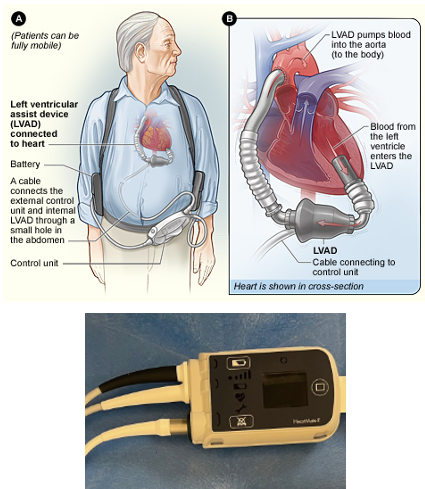
Figure 6. Examples of LVADs.
The top photo shows a diagram of the pump inside the heart connected to the outside controller. The bottom photo is a picture of the HeartMate 2 controller, which HeartMate 3 looks almost exactly like.
Education Videos
Here are several videos where patients describe their experiences living with an LVAD.
This can be a helpful resource for both us as practitioners and our patients who may be hospitalized in the cardiac ICU awaiting LVAD implant. They may be feeling anxious or uncertain about their upcoming procedure, and this is a major life change that's about to occur. So, perhaps just helping patients cope and watching these videos can help.
OT Considerations
There are some things to consider when treating patients with an LVAD. LVADs need a power source at all times for the pump to run. Here are some images in Figure 7.

Figure 7. LVAD controller and batteries.
The top photo visually overviews the LVAD controller and its essential components. Directly next to the controller are two rectangular batteries, which are fastened into battery clips and connected to two power cables—typically one white and one black. These cables are crucial for powering the device. The additional white cord shown in the image, which is partially cut off, is the driveline. This cord exits through the patient’s abdomen and connects to the internal heart pump. The bottom photo shows the charging station, where four batteries should always be charging to ensure continuous access to power for the patient.
These batteries are used when the patient moves away from their primary living space, commonly called their “home base,” such as a bedroom. When at home or in a hospital room, the LVAD is typically connected to wall power through those same two power cables. A critical point for occupational therapy practitioners to understand is that only one power source should be disconnected when switching the LVAD between wall and battery power. We should never attempt to disconnect or connect these power cables unless properly trained. Some institutions require that a nurse supervise this action, and each facility will have its protocol. Knowing and following your institution’s guidelines is essential before interacting with any LVAD equipment.
In terms of pump flow, this calculated value estimates the amount of blood being circulated out of the pump per minute. It is based on the pump’s speed and the power supplied to the motor. Each patient will have an established flow goal, such as greater than 4.6 liters per minute, typically found in the medical record or confirmed with the ICU team. Monitoring the patient’s flow, blood pressure, and overall response to activity during treatment sessions is essential for safety and appropriate clinical decision-making.
A loud, low-flow alarm will sound when the pump flow drops below 2.5 liters per minute. This may occur if the pump stops functioning, it’s not working properly, or the driveline has become disconnected from the controller. It can also be triggered by physiological changes such as drops in blood pressure. If this alarm goes off, we must intervene quickly by notifying the nurse and assisting the patient to return to bed supine with their legs elevated, similar to how we would respond to a patient experiencing orthostatic hypotension.
It’s also critical to understand that automated blood pressure monitors generally do not work on LVAD patients. Most of these patients are non-pulsatile and do not have a palpable pulse, which makes traditional cuff readings ineffective. Instead, blood pressure is measured by a nurse using Doppler ultrasound, unless the patient has an arterial line that provides continuous, real-time blood pressure monitoring. Automated monitors can only be used if the patient is pulsatile, which is not typically the case with LVAD recipients.
As with other cardiac surgeries, patients who receive an LVAD via a median sternotomy will follow sternal precautions during recovery. However, it’s worth noting that some LVADs can be implanted via thoracotomy, a less invasive technique involving a small incision in the side of the chest, which may affect the type and duration of activity restrictions.
Maintaining the safety and integrity of all LVAD components is a crucial part of our role. Before and during any activity with the patient, we should assess the driveline site and ensure the dressing is intact. It’s also important to confirm that the driveline anchor—a small adhesive device located near the exit site—is properly secured to reduce tension and prevent accidental dislodgement during movement. The external controller and batteries should be stored securely, often in a shoulder bag or vest. Immediately after surgery, most patients are provided with a shoulder bag, similar to a crossbody or messenger bag, where the components are enclosed and zipped for safety during mobilization.
If there are any concerns during treatment—whether related to equipment, alarms, or patient symptoms—we must immediately alert the nurse. Finally, when LVAD patients are cleared to leave the cardiac ICU for therapy or other activities, they should always be accompanied by a nurse and have a backup bag with them. This bag contains a backup controller, extra batteries, and any other emergency supplies they might need in case of equipment failure.
Being aware of these details and integrating them into our treatment approach ensures that we maintain the highest level of safety and preparedness while delivering functional, patient-centered care.
OT Role
When treating status post-LVAD implant patients, our role as occupational therapy practitioners centers around ensuring patient safety and promoting independence as they transition into upright activity and mobility. Although some of this may feel redundant, it’s worth reiterating that patients must move slowly, allowing their bodies time to adjust to position changes. As we guide them through mobility tasks, we must closely monitor their flow readings and ensure that any drop in flow recovers appropriately before progressing further. These early mobilization efforts typically begin with the patient in bed or in the chair position—many ICU beds have a bed-to-chair function that allows patients to gradually acclimate to being upright before fully getting out of bed.
We also want to increase the patient’s awareness of their LVAD equipment. In the early stages of recovery, understanding where the LVAD controller is located in bed or on the body is a good first step. This awareness sets the stage for eventually integrating the controller and battery management into the patient's everyday activities. Taking ownership of the equipment is an essential step toward independence.
We can also support fine motor coordination and hand strength, essential for patients to manage the LVAD hardware. Switching power sources requires twisting and pulling motions, and the batteries must be snapped into clips with a bit of force. In addition, patients may need to manage zippers, Velcro, or buckles on their LVAD shoulder bags, vests, or other adaptive garments. These tasks require adequate hand function, and we can design interventions to build the strength and coordination necessary to perform them.
Cognitive interventions also play a critical role. We can support patient independence by reinforcing their understanding of the steps required to switch from wall power to battery power and back again. As OT practitioners, we’re uniquely skilled in task analysis, so we can help patients break down this complex routine into manageable steps that they can apply to their daily lives. While the LVAD team generally initiates the education process, we are key players in reinforcing that learning and helping patients transfer these skills into meaningful routines. Our ultimate goal is to ensure that patients can independently manage power source transitions and secure their controller and batteries in their shoulder bag, vest, or belt in a way that supports optimal posture and comfort. Because the equipment adds several pounds of weight, we need to monitor for poor posture and address any musculoskeletal issues related to carrying the device.
Safety awareness remains a high priority. In addition to cognitive education around heart failure management, which we've already discussed, there are a few differences for LVAD patients. One notable distinction is that LVAD recipients must stay adequately hydrated for optimal pump function, unlike typical heart failure patients. They should be taking in approximately 2 to 2.5 liters of fluid daily. As we address health maintenance habits, we need to incorporate this specific target and reinforce adherence to blood thinners and potentially other medications like diuretics or blood pressure regulators, all of which will be part of the patient’s lifelong routine.
Bathing and hygiene routines also require adaptation. LVAD patients are typically discouraged from showering post-implant, as eliminating showers has been shown to reduce the risk of infection at the driveline exit site. OTPs help patients establish new bathing routines such as sponge bathing, bath wipes, or waterless body washes. We should always consult institution-specific protocols to confirm shower restrictions and educate accordingly.
Another area where we provide critical support is in sleep routines. Patients with an LVAD can no longer sleep on their stomachs due to the risk of compressing or tugging on the driveline, and if they have a sternal incision, that’s another area that must be protected. We can suggest pillow placement strategies to improve sleep comfort and encourage safe sleeping positions. We must also reinforce that patients must remain on wall power overnight to avoid battery depletion. Because this setup involves a long power cable, we must consider fall prevention strategies. This power cord can pose a serious tripping hazard for patients who get up frequently at night to use the bathroom.
To mitigate this risk, we might suggest using a bedside commode as a short-term solution during the early recovery phases. If a patient insists on walking to the bathroom, we can recommend placing multiple night lights along the path to illuminate the floor and help them avoid tripping over the cable. These kinds of adjustments, though seemingly small, can make a significant difference in keeping our patients safe as they adapt to living with a complex life-sustaining device. Through personalized support, adaptive techniques, and clear education, we help empower patients to regain control of their routines and safely reintegrate into daily life.
Extracorporeal Membrane Oxygenation (ECMO)
Now we're moving into our last main topic, which is the role of an occupational therapy practitioner in managing patients on ECMO in the cardiac ICU.
ECMO stands for extracorporeal membrane oxygenation. It’s an advanced critical care procedure to treat patients with severe cardiac, respiratory, or cardiorespiratory failure. ECMO specialists implement this intervention and typically provide it in high-volume ECMO centers. In the photo in Figure 8, you can see an example of an ECMO circuit.
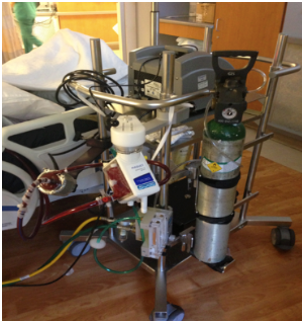
Figure 8. ECMO.
This is essentially a heart-lung machine that removes carbon dioxide and sends oxygen-saturated blood back to the tissues in the body.
It works because blood flows from the right side of the heart to the membrane oxygenator in the heart-lung machine. Once oxygenated, the blood is rewarmed and returned to the body. This process allows the blood to bypass the heart and lungs entirely, allowing these organs to rest and heal.
VA ECMO
Figure 9 shows one type of ECMO, a veno-arterial or VA ECMO.
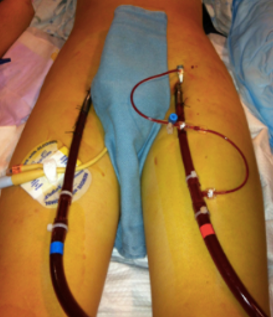
Figure 9. Veno-arterial (VA ECMO).
This is treatment for cardiogenic shock, cardiac and respiratory failure, or high-risk intervention. Cardiogenic shock is a sudden onset of impaired heart function when the heart cannot pump effectively enough to meet the body's demands. It often happens after a myocardial infarction.
With VA ECMO, a cannula is placed in a large vein to draw blood out of the body, and then it's returned through another cannula placed in a large artery. The photo here shows a bifemoral configuration, where blood is drawn from the femoral vein and returned through the femoral artery after it passes through the ECMO circuit.
VV ECMO
VV ECMO, or venovenous ECMO, is another one shown in Figure 10.
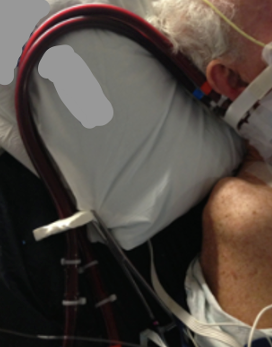
Figure 10. Veno-venous ECMO.
This is treatment for pure respiratory failure. It does not provide cardiac support. This setup can involve one double-lumen cannula placed in a single vein or two cannulas in two different veins. The photo here shows a right internal jugular vein configuration with a double-lumen cannula.
This approach can be used for potential lung transplant candidates. We also saw an increased number of patients on VV ECMO during the pandemic, particularly those with severe COVID-19. You may also see ECMO, which is described as an RVAD, or right ventricular assist device. This is used to treat severe right heart failure and typically involves a right IJ double lumen cannula, which may be used with or without the oxygenator connected.
Other variations include subclavian and central ECMO cannulation, which can be performed with an open or closed chest.
OT Considerations
So, some occupational therapy considerations for patients on ECMO include understanding and adhering to movement restrictions, which are typically based on the cannulation site. For patients with femoral cannulation, active hip flexion on the affected side is prohibited. The head of the bed must remain at less than 30 degrees of elevation, and these patients are generally placed on complete bed rest. However, some institutions have progressed toward mobilizing and even ambulating patients with femoral ECMO cannulation, so referring to your institution-specific protocols is important.
There is also research discussing a 90-degree hip flexion test performed in supine, held for 30 seconds, but I won’t go into too much detail here—know that this research exists and may influence practice in some settings. For patients with internal jugular or subclavian ECMO cannulation, shoulder flexion and abduction should be limited to less than 90 degrees, and pushing or pulling with the extremity on the cannulated side should be avoided.
Our goal is to maintain the safety and integrity of the cannulas at all times. We need to ensure cannulas are not kinked and must not become dislodged. Because of this, we must be extremely cautious and take a team approach when mobilizing these patients. When we enter our patients’ rooms, they often look like the example shown in Figure 11, fully connected to complex ECMO equipment and lines, requiring coordinated care and clear communication with the interdisciplinary team.
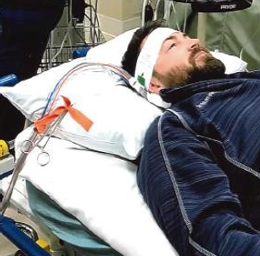
Figure 11. Someone hooked up to ECMO equipment.
One of my nursing colleagues always does a great job acting as a patient for us in the sim labs that we conduct for our nursing staff. In this example, he is on VV ECMO with right IJ cannulation. You can see the white headband around his forehead, stabilizing the cannulas as they are positioned along his neck. Patients will typically also have sutures running up the neck that help maintain the proper positioning of the cannulas.
Before we mobilize, we need to assess the cannulation site for any signs of bleeding or hematoma. We want to check that all dressings are intact and that the headband is applied securely. Anchoring devices should be in place before starting any treatment, and if there are any concerns, we need to alert the nurse immediately.
Before considering out-of-bed mobility, we must ensure the patient is ready. It’s important to remember that just because a patient is hemodynamically stable while lying flat in bed doesn’t mean they will tolerate upright activity. We should look for signs of tolerating turns in bed, head-of-bed elevation, or bed-to-chair positioning. They should also be able to tolerate routine nursing care without desaturation and with stable vital signs.
When patients have been previously cleared for out-of-bed activity and we’ve assessed them and consulted with the nurse and the ICU team, we must work very closely with all care team members to mobilize them safely. It truly takes a village. The interdisciplinary team typically includes one or more ECMO-trained nurses, possibly an ECMO champion nurse, a physical therapist, a respiratory therapist, and, in some cases, the physician, NP, or ECMO coordinator may also be involved.
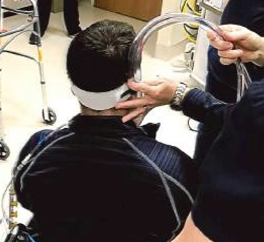
Figure 12. Nurse is stabilizing cannulas prior to a transfer.
In Figure 12, you can see my colleague again. The ECMO-trained nurse is stabilizing the cannulas and maintaining contact with his neck as he prepares to transition to standing with me for a functional transfer.
OT Role
Studies have found that delivering rehabilitation to patients on ECMO support is feasible and safe. There are actually low incidences of complications and major events during exercise. Corrick and colleagues, in their qualitative systematic review, highlighted some proposed exercise protocols with specific exercise modalities suggested for patients on ECMO. This includes active range of motion, recommending one to three sets of eight to ten repetitions daily of five different active range of motion exercises.
They also suggest passive range of motion for 20 minutes daily, which I believe is cumulative throughout the day. Active and passive cycling is another modality mentioned. This refers to an in-bed bicycle attached to the footboard of a patient’s bed. Additionally, resistive exercises, stretching, and splinting may be included, possibly to prevent contractures. Family education and training are also emphasized to promote carryover of therapy strategies outside of sessions.
Occupational therapy practitioners also play a key role in implementing cognitive interventions. These can address orientation, attention, command following, delirium prevention, and, importantly, anxiety reduction and emotional regulation. Setting expectations for patients—explaining what will happen, how we will facilitate movement to keep them safe, what will be done, when it will be done, and the sequencing of each step—can significantly reduce anxiety. I think OTs do an excellent job of breaking things down and walking patients through everything step-by-step, which helps them feel less overwhelmed.
Here’s my nursing friend again posing as our patient with VV ECMO. As always, our priority is ensuring safety. We want to mobilize these patients with a slow progression, allow for slow position changes, frequent rest periods, and reassess their ability to resume activity throughout the session. You may notice that patients on VA ECMO sometimes require shorter recovery periods—maybe around two minutes. In contrast, those on VV ECMO, who are typically dealing with severe respiratory failure, may need more extended recovery periods of five to eight minutes due to potential episodes of oxygen desaturation and fatigue.
Occupational therapy practitioners also contribute to verticalization therapy for ECMO patients, especially those unable to stand. This might involve using a tilt table or beds that can tilt and allow patients to walk off the bed. We may use a supine slide board for these sessions to help with lateral transfers from the bed to the tilt table. Our role would involve facilitating proper setup and positioning, ensuring the patient's arms are supported on the armrests, their neck is supported, their feet are properly placed on the platform, and gradually progressing the tilt. A typical progression might involve increasing the tilt by 15 degrees every two minutes while continuously monitoring the patient’s symptoms and vital signs.
Once the patient is upright, we can incorporate functional activities into the treatment session, always focusing on expectation management, emotional support, and anxiety reduction. The goal is to make the experience less overwhelming and help patients regain confidence in their ability to move and engage in their recovery.
I created this spreadsheet (Figure 13) with one of my physical therapy colleagues and just wanted to include it.
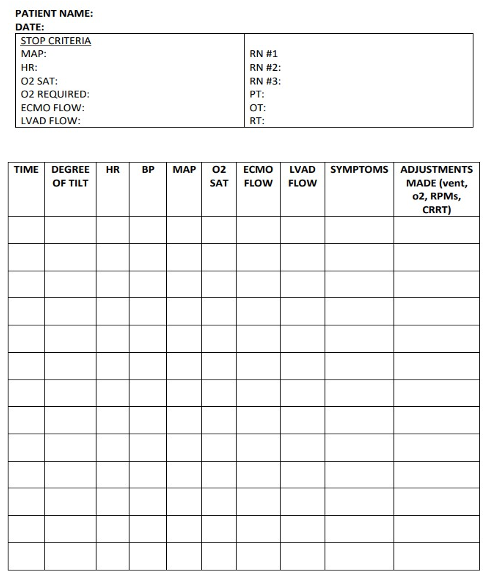
Figure 13. Author's spreadsheet.
You may find it helpful to keep a spreadsheet of vital signs when working with patients using a tilt table or any form of verticalization therapy. This can help you quickly summarize your session in your documentation and provide clear, concise feedback to the ICU team.
I also wanted to include the graphic I created (Figure 14), which shows the room setup using a tilt table.
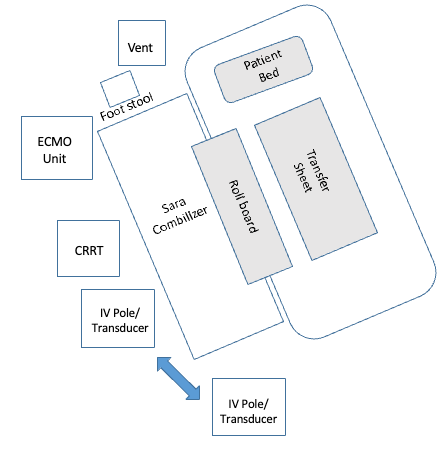
Figure 14. Example of a room set up.
We're just skimming the surface regarding verticalization therapy for this presentation. There’s a lot more detail involved in the implementation, including how to safely transfer the patient onto the tilt table and ensure the necessary team members are available to assist with equipment management. There’s quite a bit of prep time required, but it’s all worth it if our patients get the opportunity to have more upright activity in their day.
We have to ensure that our patients are safe. Another important role that we play is helping to establish a stop signal for non-verbal or intubated patients. We can support them in using dry erase boards, communication boards, adaptive call bells such as the software touch pad, and we can advocate for our speech-language pathology colleagues to be involved as well.

Figure 15. Adaptive call bell.
We also want to help them, last but not least, facilitate engagement in meaningful leisure and ADL tasks and adapt these activities based on physical and cognitive impairments and any other restrictions that they may have based on their canulation site. We also want to optimize their positioning for pressure injury prevention and edema reduction.
General OT Considerations in the Cardiac ICU
So I just wanted to include the general OT considerations in the cardiac ICU.
- Dizziness/lightheadedness
- Excessive fatigue
- Dyspnea
- Heart palpitations
- Chest pain
- Nausea/vomiting
- Sweating
- Confusion
- Anxiety/fear
- Changes in vital signs
We must be aware of the signs of activity intolerance or cardiac distress during activity, which include dizziness, lightheadedness, excessive fatigue, etc. Figure 16 shows a graphic representation of a blocked artery.
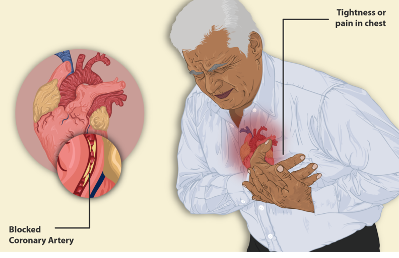
Figure 16. Example of a blocked artery.
So we must know each patient's precautions, restrictions, and activity order. This is important. We want to know the stop criteria—specifically, when we should stop based on the patient’s vital signs reaching certain values. It’s essential to be familiar with any contraindications for activity and mobility, and to monitor the patient’s vital signs before, during, and after therapy.
It’s also important to have ongoing discussions with the ICU team about the safest way to progress activity and mobility. And just as a reminder, the Borg scale can be used to help guide activity intensity, and I’ve included it again here for reference.
Lines, Tubes, Drains
I put the next five slides in for your reference (in the handout), but I wanted to move on to the case study.
Case Study: Joe
Joe is a 50-year-old male with an extensive medical history. He presented to a different hospital before coming to our hospital after he had a syncopal episode, and his implanted cardioverter defibrillator (ICD) had fired twice. So he had an extensive surgical history while he was with us. He first had an intra-aortic balloon pump placed, and then he was transferred to us. We then proceeded to remove the balloon pump and put him on femoral VA ECMO.
We gave him a temporary LVAD, which is called an Impella. His femoral VA ECMO was later decannulated, and then he was placed on right IJ VV ECMO because he became hypoxemic and required that cannulation. Several days later, he had his LVAD implanted via sternotomy, and at that point, his chest was left open. Three days later, they closed the sternum.
He was intubated and reintubated several times—a total of six times—before a tracheostomy and PEG were placed. He received an open tracheostomy and a feeding tube. Finally, he was decannulated from ECMO several days later. He had 10 OT sessions throughout a 78-day stay in the cardiac ICU. He was reevaluated by OT three times, and eight of the 10 sessions were co-evaluations or co-treatments with physical therapy. Noting some lapses in care, there were 11 days from his admission to the initial OT evaluation, and the longest lapse in care was 24 days between the first assessment and the next session, which was his reevaluation status post-VV ECMO cannulation. We have to consider why these lapses in care may occur.
An important point to consider is that in patients with extended VV ECMO runs, a study has found that when PT and OT are initiated within eight days of VV ECMO cannulation, patients do have improved functional outcomes, so this is just something to be aware of.
Some interventions while he was on bed rest with femoral VA ECMO included educating him about his restrictions and expectations for activity. We helped him participate in upper body ADLs in supine, kept the head of the bed less than 30 degrees due to restrictions at the groin site, performed range of motion exercises at bed level, and implemented delirium prevention strategies.
Once he had the right IJ VV ECMO and was cleared for upright and out-of-bed activity—clearly documented in the medical record—we facilitated gradual head of bed elevation, addressed anxiety reduction, managed expectations, reinforced energy conservation techniques, and continued delirium prevention strategies. We were able to transition him to sitting at the edge of the bed for seated ADLs, but this took a whole team approach. He had to return to supine after five minutes due to desaturation to 80% and complaints of shortness of breath and lightheadedness. We also implemented TheraBand exercises for elbow flexion and extension, provided education, and eventually facilitated safe and slow verticalization with the tilt table. We obtained a specific order from the ICU team, discussed everything with them beforehand, and proceeded with a gradual increase in tilt—15 degrees every two minutes—while continuously supporting his setup and positioning and working to reduce anxiety and manage expectations.
He tolerated 19 total tilt minutes, reaching a maximum of 55 degrees for five minutes. We continued to work with Joe even while he was intubated, providing family education on sternal precautions following his LVAD placement, teaching about the LVAD, facilitating nonverbal communication, and encouraging family participation in his exercises.
Once he had his tracheostomy and PEG and remained on VV ECMO with the LVAD, we addressed cognition, continued tilt table verticalization, and helped him safely transition to sitting at the edge of the bed. We worked on sitting balance, weight shifting, and promoting awareness of his LVAD controller, continually monitoring flows and symptoms.
After he was finally decannulated from ECMO, we continued to support him with edge-of-bed sitting and preparatory activities. We coordinated with nursing to use a mechanical overhead lift for safe transfer to a chair, where we could complete ADLs. Sit-to-stand transfers from the chair were more successful than from the bed, as he could lean back and rest between trials. Nursing assisted with trach suctioning and monitored LVAD flows and symptoms. We worked on active range of motion and stretching, particularly for left shoulder pain.
I included this reference to speech therapy’s involvement, which I found interesting. They were not utilized as fully as I would have hoped, and I think we can be strong advocates for our speech therapy colleagues if we notice that their orders have dropped off and need to be reconsulted.
Joe was eventually discharged to an LTAC—long-term acute care hospital—after his 78-day stay in the cardiac ICU. He was deemed hemodynamically stable after being off ECMO, with stable blood pressures. He was weaned off the ventilator at the LTAC and later transitioned to acute inpatient rehab for further therapy.
Summary
I hope this information was helpful to you.
References
See additional handout.
Citation
Rohrer, B. (2025). Occupational therapy practitioner's role in the cardiac ICU. OccupationalTherapy.com, Article 5809. Retrieved from https://OccupationalTherapy.com
